Abstract
Objective
We wanted to determine whether the amount and shape of the anterior mediastinal fat in the patients suffering with usual interstitial pneumonia (UIP) or nonspecific interstitial pneumonia (NSIP) was different from those of the normal control group.
Materials and Methods
We selected patients who suffered with UIP (n = 26) and NSIP (n = 26) who had undergone CT scans. Twenty-six controls were selected from individuals with normal CT findings and normal pulmonary function tests. All three groups (n = 78) were individually matched for age and gender. The amounts of anterior mediastinal fat, and the retrosternal anteroposterior (AP) and transverse dimensions of the anterior mediastinal fat were compared by one-way analysis of variance and Bonferroni's test. The shapes of the anterior mediastinum were compared using the Chi-square test. Exact logistic regression analysis and polychotomous logistic regression analysis were employed to assess whether the patients with NSIP or UIP had a tendency to show a convex shape of their anterior mediastinal fat.
Results
The amount of anterior mediastinal fat was not different among the three groups (p = 0.175). For the UIP patients, the retrosternal AP dimension of the anterior mediastinal fat was shorter (p = 0.037) and the transverse dimension of the anterior mediastinal fat was longer (p = 0.001) than those of the normal control group. For the NSIP patients, only the transverse dimension was significantly longer than those of the normal control group (p < 0.001). The convex shape of the anterior mediastinum was predictive of NSIP (OR = 19.7, CI 3.32 -∞, p < 0.001) and UIP (OR = 24.42, CI 4.06 -∞, p < 0.001).
The anterior mediastinum is mainly composed of fat tissue and it is a dynamic compartment that readily adapts to various pathologic conditions (1). Changes in the shape of the extrapleural fat and anterior mediastinum can be seen in localized inflammatory diseases such as tuberculosis and empyema, and this can also occur as a consequence of chronic fibrotic diseases of the lung and pleura (2-4). Radiation fibrosis or lobectomy can also cause focal or diffuse thickening of the mediastinum (4, 5). We have recently observed in our clinical practice that the mediastinal fat in patients with usual interstitial pneumonia (UIP) or nonspecific interstitial pneumonia (NSIP) was thicker than that in normal individuals and it tended to be convex in shape. Idiopathic pulmonary fibrosis is known to cause mediastinal widening and it can be mistaken for lymphadenopathy caused by tumor or infection (4). To the best of our knowledge, CT analysis of the amount and shape of the anterior mediastinal fat in patients with UIP or NSIP have not been described previously.
The purpose of this study was to determine whether the amount and shape of the anterior mediastinal fat in patients with UIP or NSIP are different from those of a normal control group.
From January 1999 to December 2004, we identified 43 patients with a definitive diagnosis of NSIP and 115 patients with a definitive diagnosis of UIP by searching the patient records at our hospital and at another teaching hospital. Over the same period, 252 individuals were identified who had no abnormal lesion on high-resolution CT (HRCT) and they had normal pulmonary function tests, so they were selected as controls. To avoid any bias due to age or gender, the UIP, NSIP and control subjects were individually matched for age and gender. Finally, 26 patients with NSIP, 26 patients with UIP and 26 controls were selected. Each group was comprised of 20 females and six males. For each of the three groups, the ages were matched within two years (age range: 40-77 years, mean age: 56.3).
Nonspecific interstitial pneumonia was diagnosed in 26 patients from lung wedge resections by consensus between a panel of experienced pathologists who worked in conference, and the diagnosis was based on the criteria developed by the American Thoracic Society and European Respiratory Society (ATS/ERS) (6). The histologic features of NSIP included a cellular pattern (n = 3), a fibrosing pattern (n = 10), and both patterns (n = 13). UIP was also diagnosed from lung resections in five cases or from a combination of the typical HRCT findings (as described by the ATS) and from the clinical information in 21 cases (6).
Two authors (C.H.L. and K.R.S.) reviewed the clinical histories and the underlying medical conditions. To exclude other factors that might change the amount and shape of the anterior mediastinal fat, patients with a history of tuberculosis, empyema, connective tissue diseases, exposure to organic or inorganic dust or toxic fumes, Cushing disease, asthma or any other diseases requiring steroid medication were excluded from the study population. Patients who had been treated with steroid before CT were excluded for the same reason. Our institutional review board did not require approval of our study or informed consents for the patients' records or image reviews.
High-Resolution CT was performed in all patients. The scans were 1-1.5 mm thick/section, and they were reconstructed using a high spatial frequency algorithm. Scans were obtained at 10 mm intervals in the supine position at end inspiration. The HRCT scans were obtained with a variety of scanners (Somatom Plus-4 scanner, Siemens Medical Systems, Erlangen, Germany; Somatom Plus-S scanner, Siemens Medical Systems, Erlangen, Germany; and Hi-Speed Advantage, GE Medical Systems, Milwaukee, WI). No intravenous contrast material was used.
The CT scan Digital Imaging Communications in Medicine (DICOM) format was used for the quantitative measurements. The amount of anterior mediastinal fat was determined from scans at the level of the aortic arch, and it was quantified using Rapidia software (3DMED, Seoul, Korea) (Fig. 1A). This software automatically calculates the areas of anterior mediastinal fat with the attenuation range of -190 to -50 HUs. This attenuation threshold for the anterior mediastinal fat measurement was modified from the value used for the standardized abdominal fat measurement on CT by Yoshizumi et al. (7).
The shape of the anterior mediastinal fat was categorized as concave, flat or convex at the level of the main pulmonary trunk rather than determining the shape of the anterior mediastinal fat at the level of aortic arch because the amount of anterior mediastinal fat at the level of aortic arch was insufficient for the purposes of classifying it in some cases. The anteroposterior (AP) (from the posterior wall of the sternum to the anterior wall of the ascending aorta) and transverse dimensions (the width of the posterior wall of the sternum in contact with the anterior mediastinal fat) of the anterior mediastinal fat at the level of the main pulmonary trunk were also measured (Fig. 1B). Two radiologists evaluated the shape of anterior mediastinal fat by consensus (Fig. 2).
All the analyses were performed with SAS System software (version 9.0, SAS Institute, Cary, NC). The 26 1:1:1 matched sets were analyzed, and the amounts of anterior mediastinal fat, the retrosternal AP and transverse dimensions of the anterior mediastinum, and the weights and body mass indexes (BMIs) were compared using oneway analysis of variance (ANOVA). Bonferroni post hoc test was also performed. The shapes of the anterior mediastinal fat were compared using the Chi-square test. The conditional exact logistic regression model was used to assess whether convexity of the anterior mediastinum, as determined by CT, was a risk factor for pulmonary fibrosis. The potential confounding factors including weight, BMI, the AP and transverse dimensions and the amount of fat in the anterior mediastinum, were categorized (the weight was divided for 10 kg intervals and the other factors were divided into four groups in reference to the control). Because weight was the most important confounding factor, the crude odds ratios and adjusted odds ratios for weight with the corresponding 95% confidence intervals (CI) were calculated. A polychotomous logistic regression test model was used to calculate the overall p-values for the UIP, NSIP and control groups. P values of < 0.05 were considered to indicate statistically significant differences.
Comparisons of the 78 matched study subjects are summarized in Table 1. No significant differences in age, weight and BMI were observed among the three groups. The amount of anterior mediastinal fat was not different among the three groups (p = 0.175). The retrosternal AP dimension (p = 0.037) and transverse dimension (p < 0.001) of the anterior mediastinal fat were significantly different among the three groups. Bonferroni post hoc test showed that the UIP retrosternal AP dimension was shorter (p = 0.037) and the transverse dimension was longer (p = 0.001) than that in the normal control group. For the NSIP group, only the transverse dimension was significantly longer than that in the normal control group (p < 0.001). However, no significant difference in the AP and transverse dimensions of the anterior mediastinal fat was observed between the NSIP and UIP groups.
The shapes of anterior mediastinum were significantly different among the three groups (p < 0.001) (Table 2). The convex shape of the anterior mediastinum was predictive of NSIP (OR = 19.7, CI 3.32-∞, p < 0.001) and UIP (OR = 24.42, CI 4.06-∞, p < 0.001). When the data were adjusted for weight, a convex shape was also predictive for NSIP (adjusted OR = 17.16, CI 2.89-∞, p < 0.002) and UIP (adjusted OR = 32.64, CI 5.71-∞, p < 0.002). The UIP patients were also found to have a higher likelihood of a convex shape of the anterior mediastinum than were those patients with NSIP (OR = 3.96, CI 0.74-39.79, p < 0.001; adjusted OR = 6.11, CI 0.82-275.26, p < 0.002).
Although mediastinal widening in idiopathic pulmonary fibrosis have been previously described, no objective CT analysis has been reported (4). Thus, this is the first study to investigate the use of CT images to quantitatively and qualitatively analyze the mediastinal morphologies in pulmonary fibrosis.
The mediastinum is composed primarily of fatty tissue that directly contacts the lungs bilaterally. Therefore, the shape of the mediastinum readily adapts to the changes in lung pathology (1). Mediastinal widening can be seen in the setting of idiopathic pulmonary fibrosis (4). According to the results of our study, idiopathic interstitial pneumonias such as UIP and NSIP can change the shape of the anterior mediastinal fat.
Mediastinal changes have been reported in various pathologic conditions. Mediastinal lipomatosis refers to the accumulation of excess fat, and this is usually associated with corticosteroid administration (8, 9). For those patients with UIP, the first line treatment is corticosteroid; thus, mediastinal widening can be the result of steroid medication or fibrous scarring. However, because we excluded the patients who had previously received corticosteroid medications, its effect could be ignored in the present study.
In patients with a treated neoplasm, any mediastinal bulge that develops suggests extension or recurrence of tumor. However, if the patient has been irradiated, then mediastinal widening can be due to adjacent lung scarring, which retracts the mediastinal pleura laterally and draws in fat. Other causes of lung scarring can similarly widen the mediastinum (4). For example, localized lung or pleural scarring caused by lung infection can tether the pleura and thicken the overlying fat in the chest wall or mediastinum (2, 3). The acquired causes of mediastinal rotation include lung resection and atelectasis (4). Proto (1) have also reported that a retrosternal band is a common finding on the lateral radiographs of patients with an abnormally low lung volume (congenital or acquired) on one side. Retrosternal soft tissues develop because the diminished lung volume pulls the mediastinum toward the affected side and this draws mediastinal fat anterolaterally in front of the lung. Before the advent of CT, this retrosternal band was attributed to excessive areolar tissue or to an accessory hemidiaphragm (10), but CT has shown that the actual cause of this retrosternal band is mediastinal rotation and mediastinal fat displacement (11).
We measured the amount of mediastinal fat at the level of the aortic arch, which is where most of the fat tissue can be consistently observed on HRCT. However, for classifying the shape of anterior mediastinum into three types, we selected the level of the main pulmonary trunk because the shape of the anterior mediastinum could be more objectively and consistently determined. In some cases, the retrosternal space was so narrowed that the shape of the anterior mediastinum could not be determined at the level of aortic arch.
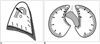
Our results showed that UIP tends to be associated with a reduced AP dimension and an increased transverse dimension with the resultant convex shape of the anterior mediastinum. In UIP patients, honeycombing change with volume loss usually occurs at the periphery of the lung base. We can speculate that the bilateral tensile force exerted by the subpleural fibrosis of the lung adjacent to the mediastinum could result in widening of the transverse dimension and shortening of the AP dimension of the anterior mediastinum (Fig. 3).
Toei at al. (5) analyzed the changes in the amounts and distributions of the anterior mediastinal fat after left upper lobectomy, as determined by CT. The postoperative anterior mediastinal fat distribution was distinctly changed with showing a marked increase from the aortic arch to the main pulmonary arterial level, with a converse decrease at the upper and lower slices, but no significant postoperative change was noted in the total anterior mediastinal fat volume. Likewise, in our study, the amounts of anterior mediastinal fat in the cases of NSIP and UIP were not significantly different from the controls. This indicates that lung fibrosis did not induce a quantitative increase of the anterior mediastinal fat, but rather that it induces a redistribution of the mediastinal fat. The loss of volume of both lower basal lungs due to the fibrosis in UIP might cause a redistribution of fat tissues and the anterior mediastinal shape changes.
Several studies have found that considerable overlap exists between the thin section CT patterns of NSIP and UIP patients, i.e., up to one-third of NSIP patients were found to have CT patterns that were similar to those of UIP patients (12-14). However, these radiologic findings were solely concerned with the intrapulmonary manifestations. Even the pathologic differential diagnosis of UIP and fibrotic NSIP is not straightforward; therefore, the current gold standard involves a clinicopathologic work-up by the physicians and radiologists, with a subsequent review of the final clinicopathologic diagnosis (6).
The prognosis of the NSIP subgroups differs; cellular NSIP responds rather well to corticosteroid treatment and it has a better prognosis than fibrotic NSIP (15-17). Patients with UIP show a more favorable prognosis than those without honeycombing (18). Therefore, we can also hypothesize that the prognosis of idiopathic pulmonary fibrosis correlates well with the progression of pulmonary fibrosis. However, measuring the extent of fibrosis and the volume loss of the lung parenchyma in UIP patients is not so easy, and there is no widely accepted standardized scoring system (19). Honeycombing represents end-stage fibrosis, but the ground-glass opacity associated with honeycombing can also be caused by interstitial fibrosis (20-22). Moreover, some ground-glass opacities disappear on the follow up studies for the NSIP patients. Thus, there are problems for how to measure the extent of overall fibrosis in idiopathic pulmonary fibrosis.
CT analysis of a dynamic mediastinum might reflect the overall amount of fibrosis in lungs because the mediastinum tends to change its shape and adapt to various thoracic conditions. Our results showed that a convex shape of the anterior mediastinum was due to a reduced AP dimension, and an increased transverse dimension tends to indicate a risk of NSIP and UIP, that is, the progression of fibrosis. Though the evaluation of the mediastinum shape is not presented as a critical method or as a definitive tool for diagnosing any specific disease entity that causes pulmonary fibrosis, it could provide a straightforward and convenient way of observing the overall fibrosis in the lung parenchyma, and it could be an indirect means of determining whether the radiologic findings are transient or not. Whether the convexity of the anterior mediastinum correlates well with the prognosis of idiopathic pulmonary fibrosis is not known, but this could be elucidated in a larger study.
There were limitations to our study. The confidence interval of our logistic regression model was excessive and this may have been caused by the limited sample size. Another limitation was that other conditions such as emphysema, chest wall movement and the phase of respiration, but not age and gender, might have affected the measurement of the anterior mediastinal fat, although inspiratory HRCT scans were performed.
In summary, the retrosternal AP and transverse dimensions of UIP patients differed from those of the normal individuals, whereas the amounts of anterior mediastinal fat were similar. UIP and NSIP patients have a tendency to have a convex shape of their anterior mediastinal fat.
Figures and Tables
Fig. 1
The method of measurement for the amount and the anteroposterior and transverse dimensions of the anterior mediastinal fat.
A. The amount of anterior mediastinal fat was quantified at the level of the aortic arch with software (Rapidia; 3DMED, Seoul, Korea).
This software automatically calculated the areas of the anterior mediastinal fat having the attenuation range -190 to -50 HUs when the region of interest was drawn.
B. The anteroposterior and transverse dimensions of the anterior mediastinal fat were measured at the level of the pulmonary trunk.
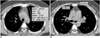
Fig. 2
The shapes of anterior mediastinal fat in the usual interstitial pneumonia, nonspecific interstitial pneumonia and normal control groups.
A, B. The concave shape of the anterior mediastinum in the normal controls.
C, D. The flat shape of the anterior mediastinum in the nonspecific interstitial pneumonia group.
E, F. The convex shape of the anterior mediastinum in the usual interstitial pneumonia group.
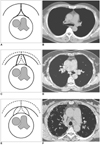
Fig. 3
A hypothetical mechanism for the morphologic change in the anterior mediastinum due to pulmonary fibrosis in the usual interstitial pneumonia and nonspecific interstitial pneumonia patients. The widening of the transverse dimension and the shortening of the anteroposterior dimension of the anterior mediastinal fat seem to be caused by bilateral tensile forces induced by subpleural fibrosis of the lung tissue that was adjacent to the mediastinum.
References
1. Proto AV. Conventional chest radiographs: anatomic understanding of newer observations. Radiology. 1992. 183:593–603.
2. McLoud TC, Isler RJ, Novelline RA, Putman CE, Simeone J, Stark P. The apical cap. AJR Am J Roentgenol. 1981. 137:299–306.
3. Im JG, Webb WR, Han MC, Park JH. Apical opacity associated with pulmonary tuberculosis: high-resolution CT findings. Radiology. 1991. 178:727–731.
4. Fisher ER, Godwin JD. Extrapleural fat collections: pseudotumors and other confusing manifestations. AJR Am J Roentgenol. 1993. 161:47–52.
5. Toei H, Furuse M, Shinozaki T, Sohara Y. Computed tomography analysis of the anterior mediastinal fat after upper lobectomy. Invest Radiol. 1997. 32:174–179.
6. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med. 2002. 165:277–304.
7. Yoshizumi T, Nakamura T, Yamane M, Islam AH, Menju M, Yamasaki K, et al. Abdominal fat: standardized technique for measurement at CT. Radiology. 1999. 211:283–286.
8. Teates CD. Steroid-induced mediastinal lipomatosis. Radiology. 1970. 96:501–502.
9. Koerner HJ, Sun DI. Mediastinal lipomatosis secondary to steroid therapy. Am J Roentgenol Radium Ther Nucl Med. 1966. 98:461–464.
10. Felson B. Pulmonary agenesis and related anomalies. Semin Roentgenol. 1972. 7:17–30.
11. Ang JG, Proto AV. CT demonstration of congenital pulmonary venolobar syndrome. J Comput Assist Tomogr. 1984. 8:753–757.
12. Johkoh T, Müller NL, Cartier Y, Kavanagh PV, Hartman TE, Akira M, et al. Idiopathic interstitial pneumonias: diagnostic accuracy of thin-section CT in 129 patients. Radiology. 1999. 211:555–560.
13. MacDonald SL, Rubens MB, Hansell DM, Copley SJ, Desai SR, du Bois RM, et al. Nonspecific interstitial pneumonia and usual interstitial pneumonia: comparative appearances at and diagnostic accuracy of thin-section CT. Radiology. 2001. 221:600–605.
14. Hartman TE, Swensen SJ, Hansell DM, Colby TV, Myers JL, Tazelaar HD, et al. Nonspecific interstitial pneumonia: variable appearance at high-resolution chest CT. Radiology. 2000. 217:701–705.
15. Daniil ZD, Gilchrist FC, Nicholson AG, Hansell DM, Harris J, Colby TV, et al. A histologic pattern of nonspecific interstitial pneumonia is associated with a better prognosis than usual interstitial pneumonia in patients with cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med. 1999. 160:899–905.
16. Flaherty KR, Travis WD, Colby TV, Toews GB, Kazerooni EA, Gross BH, et al. Histologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med. 2001. 164:1722–1727.
17. Travis WD, Matsui K, Moss J, Ferrans VJ. Idiopathic nonspecific interstitial pneumonia: prognostic significance of cellular and fibrosing patterns: survival comparison with usual interstitial pneumonia and desquamative interstitial pneumonia. Am J Surg Pathol. 2000. 24:19–33.
18. Jeong YJ, Lee KS, Müller NL, Chung MP, Chung MJ, Han J, et al. Usual interstitial pneumonia and non-specific interstitial pneumonia: serial thin-section CT findings correlated with pulmonary function. Korean J Radiol. 2005. 6:143–152.
19. Best AC, Lynch AM, Bozic CM, Miller D, Grunwald GK, Lynch DA. Quantitative CT indexes in idiopathic pulmonary fibrosis: relationship with physiologic impairment. Radiology. 2003. 228:407–414.
20. Müller NL, Staples CA, Miller RR, Vedal S, Thurlbeck WM, Ostrow DN. Disease activity in idiopathic pulmonary fibrosis: CT and pathologic correlation. Radiology. 1987. 165:731–734.
21. Müller NL, Miller RR, Webb WR, Evans KG, Ostrow DN. Fibrosing alveolitis: CT-pathologic correlation. Radiology. 1986. 160:585–588.
22. Remy-Jardin M, Giraud F, Remy J, Copin MC, Gosselin B, Duhamel A. Importance of ground-glass attenuation in chronic diffuse infiltrative lung disease: pathologic-CT correlation. Radiology. 1993. 189:693–698.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download