Abstract
Objective
We wanted to report our experience of metallic stent placement after insufficient balloon dilation in graft hemodialysis patients.
Materials and Methods
Twenty-three patients (13 loop grafts in the forearm and 10 straight grafts in the upper arm) underwent metallic stent placement due to insufficient flow after urokinase thrombolysis and balloon dilation. The indications for metallic stent deployment included 1) recoil and/or kinked venous stenosis in 21 patients (venous anastomosis: 17 patients, peripheral outflow vein: four patients); and 2) major vascular rupture in two patients. Metallic stents 8-10mm in diameter and 40-80 mm in length were used. Of them, eight stents were deployed across the elbow crease. Access patency was determined by clinical follow-up and the overall rates were calculated by Kaplan-Meier survival analysis.
Results
No procedure-related complications (stent fracture or central migration) were encountered except for a delayed Wallstent shortening/migration at the venous anastomosis, which resulted in early access failure. The overall primary and secondary patency rates (±standard error) of all the vascular accesses in our 23 patients at 3, 6, 12 and 24 months were 69%±9 and 88%±6, 41%±10 and 88%±6, 30%±10 and 77%±10, and 12%±8 and 61%±13, respectively. For the forearm and upper-arm grafts, the primary and secondary patency rates were 51%±16 and 86%±13 vs 45%±15 and 73%±13 at 6 months, and 25%±15 and 71%±17 vs 23%±17 and 73%±13 at 12 months (p = .346 and .224), respectively.
Percutaneous transluminal angioplasty (PTA) has become the first choice for treating most of the dysfunctional vascular access sites in hemodialysis patients. The technical success rate for PTA in the polytetrafluoethylene (PTFE) graft patients has been reported to be 75-94% (1-4). The randomized, prospective studies that have compared stents and angioplasty for treating dialysis graft vein stenosis have suggested that no benefit is gained by routine stent deployment (5, 6), but PTA alone was not considered appropriate to treat some types of vascular lesions that are seen in hemodialysis patients, including recoil or kinked stenosis (7-9) and venous rupture (10-12). The deployment of a metallic stent across the elbow crease has been reported on (13, 14) and different conclusions have been reached concerning the access patency for forearm and upperarm grafts (14, 15). Herein, we report our experience concerning 23 hemodialysis graft patients with metallic stent placement after insufficient balloon dilation, and all the procedures were done in a single institute over a 6-year period. We report on the overall access patency rate of our 23 dialysis-graft patients and also the feasibility of performing stent placement across the elbow crease, and we compared the results between the upper arm grafts and the forearm grafts.
From December 1998 to February 2005, a total of 1961 angioplasty procedures for dysfunctional hemodialysis shunts (approximately one half being PTFE grafts) were performed in our department. Of them, twenty-three dialysis graft patients underwent metallic stent placement to treat their peripheral venous lesions. There were 11 male and 12 female patients with ages that ranged from 51 to 85 years (mean age: 71 years). The age of the prosthetic graft at the time of stent placement was available for 20 patients (87%), with the average age being 12.2 months (range: 1-31 months). Thirteen grafts were in a loop configuration in the forearm region and the other 10 grafts were straight in shape in the upper arm. Twenty-one patients (91%) had thrombosed grafts at the time of intervention. Prior percutaneous angioplasty for these accesses (1-8 times) had been performed in 19 patients (83%) before stent placement.
Percutaneous restoration of the thrombosed graft was performed by employing our modified technique with infusing 180,000 units of urokinase (Abbokinase; Abbott Phamacoceuticals, North Chicago, IL) mixed with 2000 units of heparin in 100 ml normal saline at a rate of 75-100 ml/hr into the graft via the side port of a 7F vascular sheath. The balloon dilations that were done on the venous anastomosis and the stenotic segments along the peripheral outflow veins were performed immediately after the initiation of urokinase infusion. The arterial inflow was usually established only after the outflow venous patency was confirmed by fluoroscopy with injecting a small amount of contrast medium via the vascular sheath. Urokinase was then infused from either the angio-catheter (RC1 catheter, Cook or Bard) that was placed at the arterial limb proximal to the graft and close to the arterial anastomosis or from the vascular sheath that was placed in the graft during the later balloon dilation for declotting of any residual thrombus in both the venous and arterial systems in the graft-limb. Angioplasty for the vascular stenosis was performed using a 6-8×40 mm balloon catheter (Tru-Trac; Bard, Covington, GA) that was inflated to 8-16 atmospheres. Any stenosis that was resistant to conventional balloon dilation was further dilated with using a high pressure balloon catheter (BlueMax; Medi-tech, Watertown, MA) inflated up to 25-28 atmospheres. Recoil stenosis was dilated with a larger balloon (2 mm larger in diameter than the initial one). Any residual arterial plug at the arterial limb of the graft after balloon catheter declotting was further mechanically declotted by using a Fogarty balloon catheter (Biosensors, Newport Beach, CA). Vascular leakage was managed by prolonged low-pressure balloon inflation (5-10 min). After percutaneous restoration, a digital subtraction angiogram was repeated to assess the result of the procedure.
The indications for metallic stent placement in this study included: 1) recoil or kinked residual stenosis of at least 50% (seven patients) or > 30% residual stenosis with early re-occlusion of the graft within a one month interval (14 patients). Seventeen lesions were at the venous anastomosis (eight forearm grafts and nine upper-arm grafts), while four lesions of the forearm grafts were in the downstream outflow veins. The recoil stenosis in one brachial vein was shown to be compressed by the accompanying brachial artery (Fig. 1) on the ultrasound images, and two major vascular leaks after balloon dilations occurred in two upperarm grafts with a large pseudoaneurysm (Fig. 2) and a hematoma formation compromising the outflow vein in one patient each. Wallstent (8-10 mm×40-80 mm, Boston Scientific; Galway, Ireland) was used in our first eight patients, while nitinol stents (8 mm×40-80 mm, Memotherm/Luminexx, Bard, Angiomed, Karlsruhe, Germany) were deployed thereafter in the other 15 patients. Of them, eight stents (including four Wallstents and four nitinol stents) were deployed across the elbow crease while the other 15 stents were deployed in the upper arm region. The stents were usually deployed with overlapping of the graft matrix for 1-1.5 cm for the venous anastomotic lesions. The choice of stent was adapted to the diameter of the blood vessel (usually 1-2 mm larger than that of the adjacent vessel) and according to the stent size that was available at that time in our department.
In our study, 2,000 units of heparin were intravenously administered before the procedure. Conscious sedation was achieved by using fentanyl citrate (Sublimaze; Abbott Laboratories, North Chicago, IL) and midazolan hydrochloride (Versed Roche Pharmaceuticals, Manati', PR). Intravenous antibiotic infusion of cephazolin (1 gram) was routinely given before the procedure for the thrombosed graft accesses. No anticoagulant was prescribed for patients after stent placement. An informed consent was obtained from each patient. This study was approved by the Institutional Review Board of our hospital.
The patients were followed up by Doppler color ultrasound and /or telephone communication so that the patients who did not experience shunt failure would't have to undergo repeated angiography. All complications of the stent placement (including the eight stents placed across the elbow crease) were recorded. Technical success was defined as achieving successful hemodialysis for at least one time after the interventional procedure. Primary patency was defined as the period from stent deployment until any intervention for the vascular access was performed. Secondary patency was defined as the length of time from stent placement until the access site was lost, irrespective of the number of interventions. Patients were considered to be lost to follow-up when the patients died with a patent access. Kaplan-Meier survival analysis was used to calculate the access patency rates. Log rank testing was used to determine the difference of patency between the upper arm grafts and the forearm grafts. A p value < .05 was considered as statistical significant.
Technical success was achieved in all patients with a total of 23 initially deployed stents. No immediate procedure-related complications occurred. At the time of data analysis (April 2005), ten vascular accesses were still functioning with the average follow-up period being 19.2 months (range: 3-50 months). Eight patients died of causes unrelated to the procedure with the accesses considered patent at 3-34 months after the initial stent placement. The other five accesses were abandoned and these patients underwent surgical recreation at 2-57 months (mean period: 14.8 months). Within the follow-up period, 46 episodes of re-stenosis/occlusion occurred in 18 patients during 1-57 months of the follow-up period (mean period: 15.9 months). Of them, five patients developed new stenosis at a site away from the stent. A second stent was placed to maintain the access patency in one patient for a recurrent problem in the previously stented segment. The other episodes of restenosis/occlusion were managed by simple balloon dilations. Early re-occlusion (≤1 month) of the access after stent placement occurred in five patients. Of them, three accesses were abandoned within a very short time (1-2 months), while the other two patients still have functioning accesses for 11 and seven months, respectively, until the end of the study.
One patient with venous anastomotic recoil (an upper arm graft) had dislodgement of the deployed stent, which caused early lost of his access (Fig. 3). No other stent migrations or fractures (including the eight patients with stents across the elbow crease) were encountered during the follow-up period.
The overall primary patency rates (± standard error) of the vascular access in our 23 patients at 3, 6, 12 and 24 months were 69%±9, 41%±10, 30%±10 and 12%±8, respectively. The secondary patency rates of the vascular access at the same periods were 88%±6, 88%±6, 77%±10, and 61%±13, respectively (Fig. 4). For the forearm and upperarm grafts, the primary and secondary patency rates were 51%±16 and 86%±13 vs 45%±15 and 73%±13 at 6 months, and 25%±15 and 71%±17 vs 23%±17 and 73%±13 at 12 months (p = .346 and .224), respectively. A large pseudoaneurysm occurred in one of our patients who had a poor response to both prolonged balloon inflation and bare nitinol stent placement, and the anemysm was completely occluded after two sessions of balloon inflations (5 minutes each) within the stent (Fig. 2). The two patients with vascular rupture maintained good primary patency of their accesses for 13 months and six months, respectively, till the end of the study.
A high technical success rate (75%-94%) for pharmacomechanical thrombolysis has been reported for restoring the patency of an occluded dialysis graft (3). The persistent narrowing (recoil stenosis) at the venous anastomosis after balloon angioplasty is estimated to occur in 1.6%-15% of the upper extremity grafts (9) while the incidence of venous rupture during balloon dilation was reported to be up to 20% (16). Both recoil stenosis and venous rupture may result in PTA failure or the early re-occlusion of the vascular access. In our series, we also found brachial artery compression to be one of the causes of a stenotic outflow vein. This phenomenon does not seem to be previously mentioned in the literature, and its prevalence is unclear. Further large series concerning the imaging correlation of the venographic and ultrasonographic findings is warranted in the subgroup of patients treated with brachio-brachial shunt. Metallic stent placement is indicated to restore and maintain the vascular access patency (7-16), although metallic stent placement across the venous anastomosis precludes surgical revision of patch angioplasty, which represent only a minority (12-18%) of the surgical graft salvage procedures due to it conferring a lower cumulative graft patency (9, 17).
The overall 1-year primary patency rate in Vogel's series (53 graft patients with peripheral lesions) was 20% (15), and the secondary patency rate of Patel's study (26 patients with Wallstent across the venous anastomosis) was 50% at one year (9). In our study, the 1-year primary and secondary patency rates of our 23 patients were 30% and 77%, respectively. These rates were slightly superior to that of both the series mentioned above, and they were comparable to those rates to the Funaki's series (8) of 20 patients with thigh grafts (a 1-year secondary patency rate of 81%).
Kolakowski et al. (14), in a retrospective study, reported a poorer primary patency for forearm grafts as compared with upper arm grafts (0% vs 17%, respectively, at 1-year), and they recommended surgical thrombectomy and revision as the primary treatment for thrombosed dialysis forearm grafts. On the contrary, Vogel and Parise (15) have found the that mean period of patency of forearm grafts was 17.9 months after peripheral stent placement, compared with an upperarm graft patency period of 6.7 months (p = .014). In our study, although the number of patients was small, both the primary and secondary patency rates of the forearm and upper arm grafts showed no statistical significance. One reason for the poorer patency in Kolakowski's series may be attributed to the selection of stents. They adopted a shorter stent (2-4 cm in length) to treat the venous lesions, while stents 4-8 cm in length were used in our patients, and a mean stent length of 5 cm was used in Vogel's series. The other possible cause was that most of the stenotic lesions occur at the venous anastomosis, and in Kolakowski's series, none of the stents was placed across the elbow joint, which implied that at least two stenotic lesions existed in most of their forearm graft patients.
Because of the concern for stent migration or fracture, most interventionalists are reluctant to place stents in proximity to a highly mobile joint, particularly at the elbow joint (13). In Patel's and Kolakowski's series (26 and 61 patients, respectively), no stents were placed across the elbow joint (9, 14). Vogel and Parise (15), in their recent report, placed stents in 17 patients at the anastomosis of the lower arm dialysis grafts across or adjacent to the elbow joint. They found no difference in patency between these patients and those who had stents placed at other peripheral venous sites, with only a single instance of venous dissection occurring in a patient in Vogel et al.'s study. In our series, we had placed stents across the elbow crease in eight forearm graft patients without any complication. The only complication of stent migration in our series occurred in an upper arm graft.
Vascular leak after balloon dilation occurred most frequently at the venous anastomosis of a graft, with 1.7% of the severe ruptures necessitating stent placement (10-12). In our daily practice, venous rupture after balloon dilation occurs not infrequently, but in most instances, this complication can be well managed by prolonged balloon inflation. There were only two graft patients in our series who needed stent placement for salvaging the access. This is because most of the grafts we managed were occluded at the time of intervention and we dilated the stenotic segment at the venous anastomosis or the peripheral outflow stenosis before introducing the arterial inflow. If vascular leakage is found immediately after PTA, balloon inflation for 5-10 minutes will usually seal the leak. Thus, the ensuing urokinase thrombolysis and declotting can be performed successfully. Yet for an already flow-established or stenotic dialysis access, a major vascular rupture will usually fail to respond to conventional management. In Weber's article (11), they demonstrated that one vascular rupture failed to cease bleeding after a bare metallic stent placement, and this resulted in loss of the access. Using a covered stent is an alternative to immediate halt extravasation (10, 12, 18), but this type of stent is twice as expensive and it requires the insertion of a larger vascular sheath. Combined bare stent placement and prolonged balloon dilation within the stent can be applied to these patients. Covered stents can be reserved to treat the persistent bleeding that is not controlled by the above mentioned methods.
From our present study, we consider that it is safe to place a metallic stent across the elbow joint in hemodialysis patients. But because 1) this was a retrospective study and 2) the number of patients was small, further studies with larger numbers of patients are warranted to confirm the safety and efficacy in these patients, especially in the subgroups of patients with the aforementioned specific indications. The rimary access patency rate of metallic stent placement in selected patients is not so promising, but without performing stent deployment, the vascular access will be inevitably lost in a very short time. With repeated percutaneous interventions, the secondary access patency rate in these patients is still satisfactory.
Figures and Tables
Fig. 1
A 74-year male patient with a thrombosed loop graft in the left forearm.
A. The road-map image shows that both the brachial vein (arrow) and basilic vein (arrowhead) were opacified via the downward collateral veins.
B. After balloon dilation (6 & 8 mm in diameter), a persistent narrowing (arrow) of the outflow brachial vein at the elbow region was noted. Ultrasound exam revealed that this narrowing was caused by external compression from the accompanying brachial artery (not shown).
C. An 8×60 mm nitinol Luminexx stent (arrow) was deployed at the venous anastomosis across the elbow joint. Note the crisscross appearance of the brachial vessels. A: brachial artery. V: brachial vein. E: elbow joint.
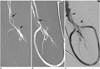
Fig. 2
A 51-year-old female patient with a thrombosed straight graft over her right upper arm.
A. A large pseudoaneurysm at the venous anastomosis after PTA was noted, which failed to response to the prolonged balloon inflation.
B. After a nitinol Memotherm stent (8×40 mm) was placed and one session of balloon inflation (5 min), small residual contrast extravasation (arrows) was still noted.
C. With a second balloon inflation in the stent for another 5 min, the follow-up venogram showed complete exclusion of the pseudoaneurysm.
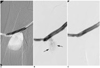
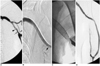
Fig. 3
A 74-year-old male patient with a thrombosed straight graft over his left upper arm.
A. Recoil (arrow) and kinked (arrowhead) stenosis at the venous anastomosis after PTA was noted.
B. A Wallstent (10×80 mm) was deployed across the venous anastomosis with overlapping of the stent with the graft matrix (arrow).
C. Stent shortening and migration caused early re-occlusion of the access one month after its placement.
D. After simple balloon dilation, the vascular flow was restored, but the access was eventually abandoned another month later.
References
1. Beathard GA. Percutaneous transvenous angioplasty in the treatment of vascular access stenosis. Kidney Int. 1992. 42:1390–1397.
2. Valji K, Bookstein JJ, Roberts AC, Oglevie SB, Pittman C, O'Neill MP. Pulse-spray pharmacomechanical thrombolysis of thrombosed hemodialysis access grafts: long-term experience and comparison of original and current techniques. AJR Am J Roentgenol. 1995. 164:1495–1500.
3. Gray RJ. Percutaneous intervention for permanent hemodialysis access: a review. J Vasc Interv Radiol. 1997. 8:313–327.
4. Turmel-Rodrigues L, Pengloan J, Bourquelot P. Interventional radiology in hemodialysis fistulae and grafts: a multidisciplinary approach. Cardiovasc Intervent Radiol. 2002. 25:3–16.
5. Quinn SF, Schuman ES, Demlow TA, Standage BA, Ragsdale JW, Green GS, et al. Percutaneous transluminal angioplasty versus endovascular stent placement in the treatment of venous stenoses in patients undergoing hemodialysis: intermediate results. J Vasc Interv Radiol. 1995. 6:851–855.
6. Hoffer EK, Sultan S, Herskowitz MM, Daniels ID, Sclafani SJ. Prospective randomized trial of a metallic intravascular stent in hemodialysis graft maintenance. J Vasc Interv Radiol. 1997. 8:965–973.
7. Vorwerk D, Guenther RW, Mann H, Bohndorf K, Keulers P, Alzen G, et al. Venous stenosis and occlusion in hemodialysis shunts: follow-up results of stent placement in 65 patients. Radiology. 1995. 195:140–146.
8. Funaki B, Szymski GX, Leef JA, Funaki AN, Lorenz J, Farrell T, et al. Treatment of venous outflow stenoses in thigh grafts with Wallstents. AJR Am J Roentgenol. 1999. 172:1591–1596.
9. Patel RI, Peck SH, Cooper SG, Epstein DM, Sofocleous CT, Schur I, et al. Patency of Wallstents placed across the venous anastomosis of hemodialysis grafts after percutaneous recanalization. Radiology. 1998. 209:365–370.
10. Raynaud AC, Angel CY, Sapoval MR, Beyssen B, Pagny JY, Auguste M. Treatment of hemodialysis access rupture during PTA with Wallstent implantation. J Vasc Interv Radiol. 1998. 9:437–442.
11. Welber A, Schur I, Sofocleous CT, Cooper SG, Patel RI, Peck SH. Endovascular stent placement for angioplasty-induced venous rupture related to the treatment of hemodialysis grafts. J Vasc Interv Radiol. 1999. 10:547–551.
12. Funaki B, Szymski GX, Leef JA, Rosenblum JD, Burke R, Hackworth CA. Wallstent deployment to salvage dialysis graft thrombolysis complicated by venous rupture: early and intermediate results. AJR Am J Roentgenol. 1997. 169:1435–1437.
13. Clark TW. Nitinol stents in hemodialysis access. J Vasc Interv Radiol. 2004. 15:1037–1040.
14. Kolakowski S Jr, Dougherty MJ, Calligaro KD. Salvaging prosthetic dialysis fistulas with stents: forearm versus upper arm grafts. J Vasc Surg. 2003. 38:719–723.
15. Vogel PM, Parise C. SMART stent for salvage of hemodialysis access grafts. J Vasc Interv Radiol. 2004. 15:1051–1060.
16. Turmel-Rodrigues L, Pengloan J, Blanchier D, Abaza M, Birmele B, Haillot O, et al. Insufficient dialysis shunts: improved long-term patency rates with close hemodynamic monitoring, repeated percutaneous balloon angioplasty, and stent placement. Radiology. 1993. 187:273–278.
17. Palder SB, Kirkman RL, Whittemore AD, Hakim RM, Lazarus JM, Tilney NL. Vascular access for hemodialysis: patency rates and results for revision. Ann Surg. 1985. 202:235–239.
18. Sapoval MR, Turmel-Rodrigues LA, Raynaud AC, Bourquelot P, Rodrigue H, Gaux JC. Cragg covered stents in hemodialysis access: initial and midterm results. J Vasc Interv Radiol. 1996. 7:335–342.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download