Abstract
Objective
To determine the utility of MR imaging in evaluating the prognostic factors for a local recurrence of rectal cancer following a curative resection.
Materials and Methods
The preoperative MR images obtained from 17 patients with a local recurrence and 54 patients without a local recurrence, who had undergone a curative resection, were independently evaluated by three radiologists. The following findings were analyzed: the direct invasion of the perirectal fat by the primary rectal carcinoma, involvement of the perirectal lymph nodes, perirectal spiculate nodules, perivascular encasement, and an enlargement of the pelvic wall lymph nodes. The clinical and surgical profiles were obtained from the patients??medical records. The association of a local recurrence with the MR findings and the clinicosurgical variables was statistically evaluated.
Results
Of the MR findings, the presence of perivascular encasement (p = 0.001) and perirectal spiculate nodules (p = 0.001) were found to be significant prognostic factors for a local recurrence. Of the clinicosurgical profiles, the presence of a microscopic vascular invasion (p = 0.005) and the involvement of the regional lymph nodes (p = 0.006) were associated with a local recurrence. Logistic regression analysis showed that the presence of perirectal spiculate nodules was an independent predictor of a local recurrence (odds ratio, 7.382; 95% confidence interval, 1.438, 37.889; p = 0.017).
Conclusion
The presence of perirectal spiculate nodules and perivascular encasement on the preoperative MR images are significant predictors of a local recurrence after curative surgery for a rectal carcinoma. This suggests that preoperative MR imaging can provide useful information to help in the planning of preoperative adjuvant therapy.
A local recurrence is the major cause of morbidity and mortality in rectal cancer patients (1). Advances in surgical techniques, including a total mesorectal excision (TME), and adjuvant therapy such as pre- and postoperative adjuvant therapy (chemotherapy and/or irradiation) have significantly lowered the rate of a local recurrence of rectal cancer (2-5). The histopathologically determined tumor stages including the depth of the tumor invasion, the presence of a regional lymph node or distant metastasis, and the presence of lymphovascular or perineural invasion are regarded to be the risk factors associated with the high recurrence rate (1, 6). However, the outcome of an individual patient cannot be predicted, and the above findings are assessable only after surgery. If the prognostic factors related to the high recurrence rate can be identified in addition to the preoperative staging, treatment planning can be tailored to each patient with a reduced probability of over- or under-treatment. The patients with such poor prognostic factors will be good candidates for preoperative neoadjuvant therapy.
The current diverse options of preoperative neoadjuvant therapy or surgical techniques require an accurate preoperative staging modality (7-9). With the advent of high-resolution techniques using phased-array coils, the role and accuracy of MR imaging in making a preoperative evaluation of rectal cancer has increased recently (10-17). MR imaging for a preoperative evaluation of rectal cancer has been used to estimate the depth of a tumor invasion (11, 15), to assess the involvement of the sphincter or pelvic structures (12, 14), and to determine the regional lymph node involvement (10, 16). Recently, Brown et al. (17) reported that MR imaging is also useful for making a preoperative assessment of the prognostic factors. However, to our knowledge, the significance of the MR imaging findings for a local recurrence has not been evaluated by a comparison with the long-term follow-up results. The aim of this study was to determine the utility of MR imaging in evaluating the prognostic factors for a local recurrence of rectal cancer following a curative resection.
Between October 1996 and December 2000, 320 rectal cancer patients, who had undergone preoperative MR imaging and a curative resection using TME, were selected from the radiological and surgical database. In our institution, preoperative pelvis MR imaging was routinely performed for the purpose of providing a staging procedure. Twenty-one patients with a local recurrence and/or distant metastasis were retrieved from those 320 patients. Four patients, who received preoperative therapy between MR imaging and surgery, were excluded in order to eliminate the effect of the preoperative adjuvant therapy on the local recurrence. Finally, 17 patients were enrolled in this study as the locally recurrent group. They consisted of nine men and eight women (mean age 59 [range 22-77] years), and had undergone a surgical resection within the two weeks of the MR imaging (mean 6 [range 2-14] days). The recurrent sites were the anastomotic sites (n=9), the pelvic sidewall (n=7), and the regional lymph node (n=1). The tumor recurrences were confirmed by open surgery (n=2), a tissue biopsy (n=9), and a clinical evaluation (n=6). The biopsy were carried out using a sigmoidoscope or colonoscope (n=7), an open biopsy (n=1), and an image guided method (n=1). The clinical diagnosis of a local recurrence was based on the follow-up CT or MR imaging showing a gradually increasing mass in the surgical area, an increased level of the serum carcinoembryonic antigen (CEA), and a worsening of the clinical course. For the purpose of comparison, the non-recurrent 54 patients (27 men and 27 women, mean age 56 years old) meeting the following criteria were retrieved: 1) they underwent preoperative MR imaging followed by curative surgery within two weeks without neoadjuvant therapy, as in the recurrent group, 2) no radiological or clinical evidence of a local recurrence or a distant metastasis for at least three years, 3) a postoperative pathological stage higher than T1 regardless of the N stage based on the TNM system (Table 1) (18), and 4) medical records with a pathological report and the MR images that could be used to review each case. Only those patients with a pathological stage higher than T1 were selected because all patients in the recurrent group had tumors higher than T1. Distant hepatic metastases were noted in two patients with a local recurrence on the preoperative MR images and a combined curative resection of the primary and metastatic lesion was performed. However, no distant metastases were noted on the preoperative MR images of the non-recurrent patients.
Based on the medical records of the subjects, the following variables from the recurrent and non-recurrent groups were compared: patients' age, gender, surgical methods, postoperative adjuvant treatment, and pathological findings from the surgical specimens, including the depth of the tumor invasion (T stage), the status of the lymph node involvement (N stage), tumor location, maximum diameter of the tumor, histological grades, the presence of a microscopic vascular invasion, and the involvement of the resection margin. The analysis was performed by dividing the surgical methods into a low anterior resection and an abdominoperineal resection, and by dividing the tumor locations into the distal, middle, and proximal regions according to the endoscopic findings. The pathological stages were divided according to the TNM system (18). The presence of a microscopic vessel invasion was determined according to the original pathological reports. The histological grades were divided into two grades: well or moderately differentiated and poorly differentiated. The circumferential resection margin was considered to be narrow when the distance from the main tumor or the mesorectal tumor deposit to the lateral surgical margin was described as being < 1 mm in the original pathological reports (19). Both findings of a narrow circumferential resection margin and the involvement of the proximal or distal margin were grouped together as an involvement of the resection margin.
MR imaging was performed on a 1.5-T whole-body system (Horizon, GE Medical Systems, Milwaukee, Wis.) using a pelvic phased-array coil. Tap water was then administered to the rectum to most patients using a rectal tube until the patient indicated discomfort. The total volume used was between 200 and 500 mL. After the localizer images were obtained, if not contraindicated, 20 mg of scopolamine butylbromide (Buscopan; Boehringer Ingelheim, Germany) was injected intravenously in order to minimize the peristalsis and to alleviate the rectal spasm.
With the patient in a feet-first, supine position, axial T1-weighted conventional spin-echo images of the pelvis were obtained using a 24 cm field of view (FOV) in a 5-mm section thickness, 1.5-mm intersection gap, 500-600/8-10 (repetition time msec/echo time msec), 256 × 192 matrix, and 1 signal acquired. The axial, sagittal, and coronal T2-weighted fast spin-echo images were then obtained using a 24-26-cm FOV in a 5-6-mm section thickness, a 1-2.5-mm intersection gap, 4,000-6,000/75-105 (repetition time msec/echo time msec), a 512 × 256 matrix, an echo train length of 10-12, and the two signals were averaged.
Three gastrointestinal radiologists (O.Y.T., L.J.S., K.J.H.), who were not involved in the preoperative interpretation of the MR examination, analyzed the hard copy images retrospectively. The MR images of the locally recurrent patients were mixed with the non-recurrent patients, and the reviewers analyzed the MR images independently in random order without the clinical or pathologic data. Each reviewer recorded all of the following items: 1) a direct invasion of the perirectal fat by the primary rectal carcinoma, 2) involvement of the perirectal lymph nodes, 3) perirectal spiculate nodules, 4) perivascular encasement, and 5) an enlargement of the pelvic wall lymph node. An interruption of the outer muscular layer and/or a grossly rounded or nodular appearance of the outer margin of the rectal mass were considered to be the indicators of a direct invasion of the perirectal fat by a primary rectal carcinoma. The direct invasion of the perirectal fat with a rounded or nodular margin, which penetrated the outer wall of the rectum, was analyzed separately again because it was believed that the two findings might have a different sensitivity and specificity for an invasion of the perirectal fat. The lymph nodes in the perirectal space was considered a metastatic lymph nodes if they exhibited the following: 1) its size was > 5 mm in the short axis diameter (20), 2) its signal intensity was heterogeneous, or 3) it had an irregular margin with a preserved nodal configuration (21). A perirectal spiculate nodule was defined as a solid nodule in the perirectal space that was separated from the primary rectal mass and had irregular spiky projections on the outer border without any configuration suggesting a nodal structure. A perivascular encasement was defined as an irregular margined soft tissue nodule or conglomerated lymph nodes that were closely attached to the branch of the perirectal vessels. The condition of the small lymph node or tumor nodule simply located adjacent to the vessel was not included in this finding. An enlargement of the lateral pelvic lymph node was defined as those cases with a lymph node > 1 cm along the pelvic wall external to the perirectal fascia (21). Each finding was defined as being present when more than two reviewers recorded the finding as being present.
The clinicosurgical profiles and MR imaging findings of the recurrent and non-recurrent groups were compared by a Student's t, a Chi-square or a Fisher's exact test. Logistic regression analysis was used to determine the independent significant factors affecting the local recurrence for the significant variables. p values < 0.05 were considered significant. A κ test was used to assess the interobserver variability in terms of the lesion detection and the differentiation of a benign lesion from a malignant focal hepatic lesion. The degree of agreement was categorized as follows: κ value of < 0, poor; κ of 0.00-0.20, slight agreement; κ of 0.21-0.40, fair agreement; κ of 0.41-0.60, moderate agreement; κ of 0.61-0.80, substantial agreement; and κ of 0.80-1.00, almost perfect agreement (22).
For the 17 patients in the recurrent group, the time interval between surgery and local recurrence ranged from five to 48 months (mean duration: 17 months). A local recurrence occurred within two years in 15 (88%) patients (mean duration: 14.6 months) and at 34 and 48 postoperative months in the remaining two patients. The follow-up periods in the non-recurrent patients ranged from 40 to 65 months (mean duration: 53 months).
For a direct invasion of the perirectal fat by the primary rectal carcinoma, when the irregularity and nodular bulging of the outer wall of the rectum were used as the criteria, the sensitivity, specificity, positive predictive value (PPV), and negative predicted value (NPV) of the preoperative MR imaging were 94%, 26%, 78%, and 63%, respectively. However, when only nodular bulging was used as the criterion, they were 48%, 84%, 89%, and 37%, respectively. For regional lymph node involvement, the sensitivity, specificity, PPV, and NPV of the MR imaging were 77%, 35%, 35%, and 77%, respectively.
Table 2 shows a comparison of the preoperative MR imaging findings between the two groups. Perirectal spiculate nodules and perivascular encasement were significantly more common in the recurrent group than in the non-recurrent group (Figs. 1, 2). Enlarged pelvic wall lymph nodes were also more commonly observed in the recurrent group, but the difference was only marginally significant (p=0.052) (Fig. 3). The κ statistical analysis results showed moderate to substantial agreement between the observers on the MR imaging findings as follows: 0.52-0.61 for outer wall penetration, of 0.38-0.49 for regional lymph node involvement, and of 0.47-0.68 for the presence of spiculate nodules.
Table 3 shows a comparison of the clinicosurgical profiles between the two groups. The microscopic vessel invasion and N stage were more common in the recurrent group than in the non-recurrent group. The lateral margin was narrow or involved in two patients from the recurrent group and in three from the non-recurrent group. Distal margin involvement was observed in two patients from the recurrent group, but in none from the non-recurrent group. Involvement of the resection margin was more common in the recurrent group, but the difference was only marginally significant (p=0.052). The other variables were not associated with a local recurrence.
Table 4 shows the results of the logistic regression analyses of the MR imaging and clinicosurgical findings. Because the seven patients with a perivascular encasement belonged to the 12 patients who had a perirectal spiculate nodule, the perivascular encasement and perirectal spiculate nodule were grouped together, and only the latter was included in the logistic regression analysis. Therefore, logistic regression analysis included the perirectal spiculate nodule, the histopathologically determined pathological node status, vascular invasion, and the involvement of the resection margin. The results showed that the presence of a perirectal spiculate nodule (odds ratio, 7.382; 95% confidence interval, 1.438-37.889; p value, 0.017) was the only variable independently predictive of a local recurrence.
The perirectal spiculate nodule observed on the MR images in this study has not been reported elsewhere. On a routine analysis of the preoperative MR images of rectal cancer, a perirectal spiculate nodule is not normally considered to be an independent finding but it might be categorized as either a metastatic lymph node or a tumor nodule of a T3 disease. It may either be a metastatic lymph node with an extranodal extension (16) or a perirectal tumor deposit described in colon cancer (18, 23, 24). Goldstein et al. (23) described the pericolic tumor deposit as a grossly palpated adenocarcinoma within the pericolic adipose tissue, but not within the lymph node, probably representing an adenocarcinoma extending along the nerves or vessels, and indicating a poor prognosis. According to new edition of the American Joint Committee on Cancer (AJCC) cancer staging handbook (18), there was some comment about the tumor nodule on the perirectal adipose tissue. A tumor nodule in the pericolorectal adipose tissue of a primary carcinoma without histological evidence of a residual lymph node in the nodule is classified in the pN category as a regional lymph node metastasis if it has the form and smooth contour of a lymph node. If the nodule has an irregular contour, it should be classified in the T category and be coded as either V1 (microscopic venous invasion) or V2 (if it was grossly evident), because there is a strong likelihood that it represents a venous invasion (18). These statements suggest that an irregular shaped tumor nodule in the perirectal space is a significant prognostic factor and should be dealt with separately. Therefore, special attention needs to be paid to perirectal spiculate nodules in MR images and it should be evaluated separately from metastatic lymph node. The perirectal spiculate nodule might indicate the biological aggressiveness of the primary carcinoma and the locally advanced disease, which requires more intensive therapy.
A perivascular encasement has also never been addressed before. In general, a vascular invasion is highly suggested when the tumor is in close contact with a vessel. Microscopic vascular invasion of a rectal tumor has been reported to be a dismal sign of an increased rate of local recurrence and poor survival (1, 6, 25, 26). Brown et al. (17) reported a tubular structure on the MR images as a gross tumoral vascular invasion. However, a gross vascular invasion can be observed in advanced diseases after a microscopic invasion progresses to the gross scale. A review of the histopathological reports, three from seven patients (43%) with the MR findings of a perivascular encasement had a microscopic vascular invasion, which is in contrast to nine out of 64 patients (14%) without them. Although a microscopic vascular invasion was more frequent in those patients with perivascular encasement, a histological vascular invasion was not always described in the pathological reports of patients with a perivascular encasement. Because the surgical specimens in this study had not been analyzed using whole mount histology with a direct radiological-pathologic correlation, the precise relationship between the perivascular encasement and the microscopic vascular invasion could not be determined. However, because perivascular encasement was closely related to the vessels and microscopic vascular invasions are more frequently reported in patients with them, it was hypothesized that perivascular encasement might be related to a vascular invasion. The radiological-pathological correlated studies should be followed in order to verify this hypothesis.
A perirectal lymph node metastasis is a well-known risk factor for a local recurrence (3, 27, 28). However, in this study, the accuracy of the preoperative MR imaging for determining the involvement of the perirectal lymph nodes was low, as in previous reports (10, 11, 13, 29). Therefore, the regional lymph node metastasis determined by the MR imaging was not associated with a local recurrence in this study, even though the nodal status determined histologically was. MR imaging cannot accurately diagnose the presence of a microscopic metastasis in the regional lymph nodes, and it is also inaccurate in estimating the nodal status based on the size criteria (10, 16). Brown et al. (16) reported that prediction of nodal involvement in rectal cancer with MR imaging could be improved by using the border contour and the signal intensity characteristics of the lymph nodes instead of the size criteria. If the encouraging criteria suggested by Brown et al. (16) are considered, the accuracy of the nodal staging with MR imaging is expected to increase.
In this study, a direct invasion of the perirectal fat by the primary rectal carcinoma, as determined by either MR imaging or the histological examination, was not associated with the local recurrence. This is to be expected because the current TME technique is useful in eliminating tumors despite their transmural extension (3, 28).
Statistical analysis of the pelvic lymph node enlargement showed a value close to statistical significance. When TME was used as the standard surgical technique, a pelvic node dissection is not usually performed. Controversy still remains as to the potential benefits of a pelvic node dissection as well its unwanted effects on voiding and sexual dysfunction (30-33). Nonetheless, a lymph node dissection or preoperative radiotherapy at the affected site is an option that can be considered when a lymph node enlargement is observed on the preoperative MR images for a reduction of a local recurrence (21).
There are many treatment options for rectal cancer patients. With the introduction of the TME technique (3) and preoperative radiotherapy (34-36), the rate of a local recurrence has been reduced significantly. Some institutes in Europe recommend the use of preoperative radiotherapy as a routine treatment modality to boost survival (34, 35). Considering the recurrence rate in those patients who underwent surgery only, preoperative radiotherapy would mean that more than 70% of the patients would receive unnecessary additional treatment. Therefore, accurate staging should precede the application of radiotherapy. Furthermore, the discrimination between locally advanced disease with a high risk of a local recurrence and localized disease with a low risk is needed. In this study, the preoperative MR image could provide not only the preoperative staging but also the potential for providing information on a local recurrence.
This study has several limitations. First, the total number of study cases and the overall number of cases with a local recurrence were small. Second, the histopathological findings of the surgical specimen were based on the original pathological reports without direct radiological-pathologic correlations. However, these findings were clinically correlated with the local recurrence by the follow-up study. These results should be verified by further studies using a large population and a pathological correlation in order for these findings to be a useful guideline for treatment. Third, the follow-up period of the patients was relatively short. However, because a local recurrence usually occurs within 2-3 years in most cases (21, 30), is believed that this limited follow-up period did not adversely affect these results.
In conclusion, the results of this study indicate that the perirectal spiculate nodule and perivascular encasement depicted by the preoperative MR images are significant predictors of a local recurrence after curative surgery on a rectal carcinoma. These MR findings may be helpful in planning the appropriate preoperative adjuvant therapy.
Figures and Tables
Fig. 1
Spiculate nodule with a perivascular encasement depicted on the preoperative MR images obtained from a 70-year-old man with recurrent rectal cancer.
A-D. (A) T1-weighted spin-echo, and (B) T2-weighted fast spin-echo images, obtained in a transverse plane show a spiculate nodule (arrowheads) partially surrounding the mesorectal vessel (thin arrows). The mesorectal vessel appears as a tiny dot with dark signal intensity. The T2-weighted fast spin-echo (C) sagittal, and (D) coronal, MR images show the primary tumor (arrows) in the rectum. The spiculate nodule (arrowheads) surrounding the mesorectal vessel (thin arrows) can also be seen.
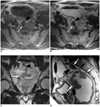
Fig. 2
Perirectal spiculate nodule depicted on the preoperative MR images (A) in a 62-year-old man and (B) in a 58-year-old woman with rectal cancer. A transverse T2-weighted fast spin-echo axial MR image shows a nodule with irregular margin (arrowheads) in the perirectal fat adjacent the primary rectal tumor (arrows).

Fig. 3
Enlargement of the pelvic wall lymph node demonstrated on the preoperative MR images obtained in a 60-year-old woman with recurrent rectal cancer.
A. A transverse T2-weighted fast spin-echo image shows a nodular tumor deposit (arrow) at the right pelvic wall out of the endopelvic fascia (arrowheads). The patient underwent a pelvic wall, lymph node dissection at the right side, and right internal iliac lymph node involvement was demonstrated.
B. Contrast-enhanced transverse CT scan obtained six months after surgery. A pelvic wall recurrence was demonstrated in the follow-up CT at the same site of the enlarged pelvic wall lymph node (arrow).

References
1. Ross A, Rusnak C, Weinerman B, Kuechler P, Hayashi A, Maclachlan G, et al. Recurrence and survival after surgical management of rectal cancer. Am J Surg. 1999. 177:392–395.
2. Tveit KM, Guldvog I, Hagen S, Trondsen E, Harbitz T, Nygaard K, et al. Norwegian Adjuvant Rectal Cancer Project Group. Randomized controlled trial of postoperative radiotherapy and short-term time-scheduled 5-fluorouracil against surgery alone in the treatment of Dukes B and C rectal cancer. Br J Surg. 1997. 84:1130–1135.
3. Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986. 1:1479–1482.
4. Reynolds JV, Joyce WP, Dolan J, Sheahan K, Hyland JM. Pathological evidence in support of total mesorectal excision in the management of rectal cancer. Br J Surg. 1996. 83:1112–1115.
5. Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001. 345:638–646.
6. Chapuis PH, Dent OF, Fisher R, Newland RC, Pheils MT, Smyth E, et al. A multivariate analysis of clinical and pathological variables in prognosis after resection of large bowel cancer. Br J Surg. 1985. 72:698–702.
7. Blumberg D, Ramanathan RK. Treatment of colon and rectal cancer. J Clin Gastroenterol. 2002. 34:15–26.
8. Minsky BD. Adjuvant therapy of resectable rectal cancer. Cancer Treat Rev. 2002. 28:181–188.
9. Petrelli NJ. Will we ever succeed in resolving the adjuvant treatment dilemma in rectal cancer? J Surg Oncol. 2003. 82:79–83.
10. Schnall MD, Furth EE, Rosato EF, Kressel HY. Rectal tumor stage: correlation of endorectal MR imaging and pathologic findings. Radiology. 1994. 190:709–714.
11. Brown G, Richards CJ, Newcombe RG, Dallimore NS, Radcliffe AG, Carey DP, et al. Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology. 1999. 211:215–222.
12. Beets-Tan RG, Beets GL, Borstlap AC, Oei TK, Teune TM, von Meyenfeldt MF, et al. Preoperative assessment of local tumor extent in advanced rectal cancer: CT or high-resolution MRI? Abdom Imaging. 2000. 25:533–541.
13. Wallengren NO, Holtas S, Andren-Sandberg A, Jonsson E, Kristoffersson DT, McGill S. Rectal carcinoma: double-contrast MR imaging for preoperative staging. Radiology. 2000. 215:108–114.
14. Urban M, Rosen HR, Holbling N, Feil W, Hochwarther G, Hruby W, et al. MR imaging for the preoperative planning of sphincter-saving surgery for tumors of the lower third of the rectum: use of intravenous and endorectal contrast materials. Radiology. 2000. 214:503–508.
15. Beets-Tan RG, Beets GL, Vliegen RF, Kessels AG, Van Boven H, De Bruine A, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet. 2001. 357:497–504.
16. Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003. 227:371–377.
17. Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003. 90:355–364.
18. Colon and Rectum. American Joint Committee on Cancer: AJCC cancer staging Handbook. 2002. 6th ed. New York: Springer-Verlag;127–138.
19. Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP. Colorectal Working Group. American Joint Committee on Cancer Prognostic Factors Consensus Conference. Cancer. 2000. 88:1739–1757.
20. Blomqvist L, Rubio C, Holm T, Machado M, Hindmarsh T. Rectal adenocarcinoma: assessment of tumour involvement of the lateral resection margin by MRI of resected specimen. Br J Radiol. 1999. 72:18–23.
21. Kim NK, Kim MJ, Park JK, Park SI, Min JS. Preoperative staging of rectal cancer with MRI: accuracy and clinical usefulness. Ann Surg Oncol. 2000. 7:732–737.
22. Kundel HL, Polansky M. Measurement of observer agreement. Radiology. 2003. 228:303–308.
23. Goldstein NS, Turner JR. Pericolonic tumor deposits in patients with T3N+MO colon adenocarcinomas: markers of reduced disease free survival and intra-abdominal metastases and their implications for TNM classification. Cancer. 2000. 88:2228–2238.
24. Harrison J, Haddad P, Maguire P. The impact of cancer on key relatives: a comparison of relative and patient concerns. Eur J Cancer. 1995. 31A:1736–1740.
25. Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. The clinical significance of invasion of veins by rectal cancer. Br J Surg. 1980. 67:439–442.
26. Petersen VC, Baxter KJ, Love SB, Shepherd NA. Identification of objective pathological prognostic determinants and models of prognosis in Dukes' B colon cancer. Gut. 2002. 51:65–69.
27. Bentzen SM, Balslev I, Pedersen M, Teglbjaerg PS, Hanberg-Soren sen F, Bone J, et al. Time to loco-regional recurrence after resection of Dukes' B and C colorectal cancer with or without adjuvant postoperative radiotherapy. A multivariate regression analysis. Br J Cancer. 1992. 65:102–107.
28. Enker WE, Thaler HT, Cranor ML, Polyak T. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg. 1995. 181:335–346.
29. Beets-Tan RG, Beets GL, van der Hoop AG, Borstlap AC, van Boven H, Rongen MJ, et al. High-resolution magnetic resonance imaging of the anorectal region without an endocoil. Abdom Imaging. 1999. 24:576–581.
30. Moriya Y, Hojo K, Sawada T, Koyama Y. Significance of lateral node dissection for advanced rectal carcinoma at or below the peritoneal reflection. Dis Colon Rectum. 1989. 32:307–315.
31. Hida J, Yasutomi M, Fujimoto K, Maruyama T, Okuno K, Shindo K. Does lateral lymph node dissection improve survival in rectal carcinoma? Examination of node metastases by the clearing method. J Am Coll Surg. 1997. 184:475–480.
32. Glass RE, Ritchie JK, Thompson HR, Mann CV. The results of surgical treatment of cancer of the rectum by radical resection and extended abdomino-iliac lymphadenectomy. Br J Surg. 1985. 72:599–601.
33. Hojo K, Vernava AM 3rd, Sugihara K, Katumata K. Preservation of urine voiding and sexual function after rectal cancer surgery. Dis Colon Rectum. 1991. 34:532–539.
34. Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997. 336:980–987.
35. Stockholm Colorectal Cancer Study Group. Randomized study on preoperative radiotherapy in rectal carcinoma. Ann Surg Oncol. 1996. 3:423–430.
36. Kaminsky-Forrett MC, Conroy T, Luporsi E, Peiffert D, Lapeyre M, Boissel P, et al. Prognostic implications of downstaging following preoperative radiation therapy for operable T3-T4 rectal cancer. Int J Radiat Oncol Biol Phys. 1998. 42:935–941.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download