Abstract
Objective
Rapid and effective hemostasis at femoral puncture sites minimizes both the hospital stay and patient discomfort. Therefore, a variety of arterial closure devices have been developed to facilitate the closure of femoral arteriotomy. The objective of this prospective study was to compare the efficacy of two different closure devices; a collagen plug device (Angio-Seal) and a suture-mediated closure device (the Closer S).
Materials and Methods
From March 28, 2003 to August 31, 2004, we conducted a prospective study in which 1,676 cases of 1,180 patients were treated with two different types of closure device. Angio-Seal was used in 961 cases and the Closer S in 715 cases. The efficacy of the closure devices was assessed, as well as complications occurring at the puncture sites.
Results
Successful immediate hemostasis was achieved in 95.2% of the cases treated with Angio-Seal, and in 89.5% of the cases treated with the Closer S (p < 0.05). The rates of minor and major complications occurring between the two groups were not significantly different. In the Closer S group, we observed four major complications (0.6%), that consisted of one massive retroperitoneal hemorrhage (surgically explored) and three pseudoaneurysms. In the Angio-Seal group, we observed three major complications (0.3%) that consisted of one femoral artery occlusion, one case of infection treated with intravenous antibiotics and one pseudoaneurysm.
Hemostasis after gaining arterial access for diagnostic and interventional catheterization has classically been accomplished by performing manual compression of the punctured artery. However, this necessitates prolonged periods of bed rest following sheath removal. In previous studies, an arterial closure device was proven to improve patient comfort, decrease the time to ambulation and shorten the patients' hospital stay as compared with simple manual compression (1-5).
Angio-Seal is an arterial closure device that involves a collagen plug and an intraarterial anchor. The arteriotomy site is positioned between them like the filling of a sandwich, and the collagen induces coagulation. This method has been proven effective in reducing the time to hemostasis and ambulation, subsequent to both transfemoral angiography and intervention (1-6). The Closer S is a kind of percutaneous suture closure device that has demonstrated a relatively high hemostasis efficacy rate (7-11). To the best of our knowledge, there have been no prospective studies that have compared the Angio-Seal and the Closer S devices. This trial is the first prospective study to compare the efficacy, device failure rates and complication rates of the two devices during both angiographic and interventional procedures.
Angio-Seal is a collagen-based vascular hemostasis device that utilizes an absorbable intravascular polymer anchor and an extravascular collagen sponge that are connected by an absorbable positioning suture. The Closer S is a suture-mediated vascular hemostasis device that consists of one nonabsorbable polypropylene surgical suture, which forms a square knot on the arterial surface.
In all the patients, the closure of femoral arteriotomy was performed in the catheterization laboratory immediately after a transfemoral interventional procedure. The exclusion criteria for the application of a closure device included difficulty in puncturing the artery, severe peripheral vascular disease, marked obesity, an age < 15 years, the arterial sheath size < 4 French or > 8 French and the patient's refusal to provide a written informed consent. In order to initiate the deployment of the device, we used a guidewire to exchange the existing sheath for a 6 French or 8 French closure sheath. While applying pressure, the device shaft was rotated into the subcutaneous tissue and then it was positioned into the artery. Once arterial placement was confirmed by pulsatile blood flow through the marker lumen, in the case of Angio-Seal, the collagen suture weave was centered and compacted onto the extravascular surface at the arteriotomy site. In the case of the Closer S, the needles of the device were deployed and they captured the sutures, which were tied with a knot-tying device called a clincher. Once the knot was preliminarily tied above the skin level, the clincher was removed and the knot was clinched down onto the artery.
All the patients receiving closure devices in this study were required to remain in a supine position in bed for two hours. However, the patients who were unable to ambulate due to their disease or their unconsciousness, or if they required subsequent surgery or procedures, or if they did not wish to ambulate, they were then kept in bed for more than two hours.
Over an 18-month period from March 28, 2003 to August 31, 2004, all the patients who underwent diagnostic angiography or endovascular interventional treatment at Samsung Medical Center (SMC) received detailed information about the closure devices prior to the procedure. Among them, 1,221 patients with 1,723 cases of diagnostic angiography or endovascular intervention provided written informed consents for the application of closure devices. However 41 patients with 47 cases of diagnostic angiography or endovascular intervention who displayed contraindications for the closure devices were excluded: 31 cases had difficulty in puncture, three had marked obesity and 13 had severe atherosclerotic stenoocclusive disease. Finally, 1,676 cases (961 with Angio-Seal, 715 with the Closer S) in 1,180 patients were enrolled in this study. The interventional radiologists and neuroradiologists at SMC had experience with 322 cases of Angio-Seal and 97 cases of the Closer S prior to beginning this study. All the enrolled patients were randomized to either the Angio-Seal group or the Closer S group at the end of the angiography procedure or after the endovascular interventional procedure.
All the patients were closely monitored for 24 hours after the procedure. After one month, they received follow-up examinations by their physician or they were contacted over the telephone to assess the status of the puncture sites.
All the definitions had been formulated in previously published studies (9, 12). Immediate hemostasis was defined as complete hemostasis after the deployment of the device, either with or without the need for additional manual compression within three minutes from the start of the procedure. Major complications were defined as follows: the need for vascular surgery, hemorrhage requiring transfusion, pseudoaneurysm, arteriovenous fistula, arterial occlusion or distal arterial embolism, and infections necessitating treatment with intravenous antibiotics or surgical debridement. Minor complications included any hemorrhage from the puncture site that was controlled via conservative management without transfusion (i.e. additional manual compression, sandbag placement or prolonged bed rest), and infections that could be treated with oral antibiotics. Moreover, all the complications were categorized as either early or late. Early complications were those that occurred within 24 hours, and late complications were those that occurred at least 24 hours after the procedure. Therefore, successful vascular device closure was defined as immediate hemostasis without complications.
The demographic and clinical outcome data were prospectively collected using a standardized "procedural data sheet for the closure device" and the data were recorded on the day that the procedure was performed, 24 hours afterward and at one month, or at the time when the complications were noted. The procedural data included the type of intervention, the procedure data, sheath size, the procedure-related drug dose and the number of previous punctures. Major or minor complications, as well as the time of events, were also recorded.
All the categorical variables were analyzed using chi-square tests and Fisher's exact tests in order to determine the statistical differences. p values < 0.05 were considered to be statistically significant.
One thousand six hundred and seventy-six femoral arterial closure devices were used in 1,180 patients, and these cases constituted the basis for this study.
The demographics regarding the two patients groups undergoing diagnostic and interventional catheterization were comparable (Table 1). The most frequent procedures were transarterial chemoembolization (TACE) for the endovascular interventional treatment and transfemoral cerebral angiography (TFCA) for the diagnostic angiography. There were no statistical differences between the two groups with regard to gender, age, the underlying disease including diabetes and hypertension, smoking or the sizes of the arterial sheaths.
Angio-Seal (n = 961) was successful in 915 cases and this corresponds to a 95.2% immediate hemostasis rate, and Angio-Seal was unsuccessful in 46 cases for a 4.8% failure rate for immediate hemostasis. Some complications occurred in 25 cases for a 2.6% complication rate, so success with the closure device was achieved in 890 cases for a 92.6% success rate. The Closer S (n = 715) was successful in 640 cases for an 89.5% immediate hemostasis rate, and the Closer S was unsuccessful in 75 cases for a 10.5% failure rate for immediate hemostasis. Some complications occurred in 12 of the Closer S cases for a 1.7% complication rate, and success of the closure device was achieved in 628 cases for a 87.8% success rate. Differences in the immediate hemostasis rates and the overall success rates between the two groups were found to be statistically significant (p < 0.05). There were no complications in the cases of immediate hemostasis failure in both groups.
When continuous bleeding occurred after device deployment, the patients were treated with standard manual compression until hemostasis was achieved, after which the patient was instructed to take five to eight hours of bed rest. Angio-Seal was associated with 46 cases of unsuccessful immediate hemostasis, and the Closer S was associated with 75 cases of unsuccessful immediate hemostasis. Most of the reasons for failure were device-related in the Angio-Seal group, whereas the failures tended to be technique-related in the Closer S group. The causes of the 121 failures are listed in Table 3. The reasons for failure were not apparent in 28 cases of the Angio-Seal group and in 11 cases of the Closer S group.
The 18 months of the study period were divided into quartiles and we analyzed the immediate hemostasis rate for the quartiles. The immediate hemostasis rates of the two devices showed a rising curve over the study period. The immediate hemostasis rate was not significantly different during the four quartile (97.8% in the Angio-Seal group and 96.1% in the Closer S group, p > 0.05), although it was significantly different during the first quartile (93.5% in the Angio-Seal group and 82.5% in the Closer S group, p < 0.05).
Twenty-five complications occurred in the Angio-Seal group. Three cases (0.3%) were major complications, and 22 cases (2.3%) were minor. Twelve complications occurred in the Closer S group, and we observed four major complications (0.6%) and eight minor complications (1.1%). Any statistically significant differences with regard to the major and minor complications between the two groups were not noted. All the major and minor complications are shown in Tables 4 and 5.
All the major complications developed at least 24 hours after the procedure. There was one case (0.1%) of local groin infection, which was a soft tissue infection with pus formation. This infection was treated successfully with intravenous vancomycin® (CJ). In one case (0.1%), the patient developed a cellulitis-associated pseudoaneurysm that was treated successfully via manual compression and intravenous cefazolin® (ChongKunDang Pharm, Seoul, Korea). In one case (0.1%), a right common femoral artery occlusion developed (Fig. 2). This patient received TACE through the right common femoral arterial puncture because of his hepatocellular carcinoma associated with liver cirrhosis. The right common artery occlusion was detected in the next month when an additional TACE procedure was attempted. Because collateral circulation had already been well developed without any symptoms, as evidenced by the right common femoral arteriogram, there was no necessity for any additional interventional procedures.
All minor complications were cases of groin hemorrhages. There were 19 early cases (2.1%) and three late cases (0.2%). All of the early complications were controlled by manual compression or prolonged bed rest. In all of the late complications, the patients developed hemorrhage within 5 days of discharge. Two patients did not warrant readmission and the hemorrhage stopped after the patient changed the dressing at his or her home. One patient visited the emergency room, but the hemorrhage stopped subsequent to manual compression, and no blood transfusion was required.
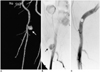
Of four major complications that occurred for the Closer S device, only one case (0.1%) was observed to develop within 24 hours. Massive retroperitoneal hemorrhage and hypovolemic shock developed. The patient underwent surgical repair and blood transfusion. Three cases (0.4%) were pseudoaneurysm cases, and one of these cases was associated with local groin infection; it was successfully treated with intravenous vancomycin® (CJ). One of these cases was treated by thrombin injection (Fig. 3), and the other was treated by manual compression.
All minor complications developed within 24 hours of the procedures. Hemorrhages developed in eight cases (1.1%). Blood transfusion was not necessary, and manual compression or prolonged bed rest proved sufficient.
In 672 cases (69.9%), the patients treated with the Angio-Seal device had undergone at least one prior femoral arterial puncture. In 234 cases (32.7%), the patients treated with the Closer S device had undergone at least one prior femoral arterial puncture. In both groups, the outcomes for these patients were similar to those of the patients who had undergone the first femoral arterial puncture (Table 6).
Prior to the advent of vascular closure devices, manual or mechanical compression with the subsequent application of a pressure bandage constituted the standard technique for the management of femoral access sites after performing diagnostic and interventional catheterizations. However, this traditional femoral access site treatment required prolonged immobilization, as well as significant patient discomfort (11). Immediate removal of the arterial sheath and achieving hemostasis of the femoral access site after such procedures tends to improve patient comfort, as well as save time, and this reduces the workload for the medical staff and reduces the length of hospital stays. Several vascular closure devices have gained market approval in the past few years. Representative of these devices are the collagen plug device called Angio-Seal and the suture-mediated device called the Closer S. To our knowledge, there hasn't been any prospective comparison trial between these two types of closure devices.
In this study, we collected prospective data to compare the outcomes of the Angio-Seal and the Closer S devices. We have demonstrated that the immediate hemostasis rates and the overall success rates of Angio-Seal were statistically higher than those of the Closer S. The incidence of immediate hemostasis was 915/961 (95.2%) in the Angio-Seal group and 640/715 (89.5%) in the Closer S group. However, the incidence of complications was 25/961 (2.6%) in the Angio-Seal group and 12/715 (1.7%) in the Closer S group. However, when comparing the immediate hemostasis rates according to the successive quartile, there was no significant difference during the fourth quartile (97.8% in the Angio-Seal group and 96.1% in the Closer S group, p > 0.05), although there was a significant difference during the first quartile (93.5% in the Angio-Seal group and 82.5% in the Closer S group, p < 0.05). The Closer S requires quite a longer time until an operator is accustomed to using the Closer S because the Closer S is more difficult and complicated to use as compared with Angio-Seal. Therefore, such disadvantage of the Closer S could decrease its efficacy at first.
The complication rates between the two groups were not determined to be significantly different. Defects in the devices themselves, as well as some variables associated with the patients, may have also had some effect on the efficacy of the two devices, although this was not analyzed in our study.
Previous studies regarding the use of compressive hemostatic techniques have reported widely varying vascular complication rates following invasive cardiac procedures, and these have been in the range of 0.2% to 10.6% (13-20). The complication rates reported by the present study are at the lower end of this range. Comparisons among these studies are confounded by interstudy variability with regard to many methodological factors, and the most important of which may be the way in which the complications are defined. For example, in one large interventional series (13), hematomas were considered to be a complication only if a 15% decrease in the hematocrit was detected, or in the cases in which surgical repair was required. In the same study, infections were considered as complications only when the patients required intravenous antibiotics or surgery. Compared with the previous studies, we used a very broad definition for complications.
A low rate of major complications was also reported in association with the use of both the collagen plug devices and the suture closure devices (12, 14, 18). In this prospective study, the incidence of major complications in the Angio-Seal group and the Closer S groups were 0.3% and 0.6%, respectively, which is well within the range reported in the published data (8-12). When comparing all the Angio-Seal and the Closer S cases, we detected no statistical differences in the major complications between the two devices. In the case of femoral arterial occlusion in the Angio-Seal group, the anchor of the device, which remains in the intraarterial lumen after the deployment of the device, may increase the risk of arterial occlusion or distal arterial embolism. Also, this can be accelerated with the application of additional manual compression. In the case of infection in the Closer S group, the patient was treated by vascular surgery, unlike the infection cases in the Angio-Seal group who were successfully treated with only intravenous antibiotics. The nonabsorbable suture material used in the Closer S system remains in the intraarterial lumen after the deployment of the device, and it may induce foreign body reactions, as well as vascular inflammation and necrosis. In the case involving massive retroperitoneal hemorrhage in the Closer S group, the patient had performed excessive hip motion of the lower extremities after being allowed to ambulate, and this motion broke the knots and suture. To prevent this accident, it is necessary to put limitations on the patient for excessive motion of the lower extremity such as performing a knee-to-the-chest posture.
The incidence of minor complications was 22/961 (2.3%) in the Angio-Seal group and 8/715 (1.1%) in the Closer S group. This was also found to not be statistically different between the two devices. These percentages are lower than those that have been reported for minor complications in the literature, which have ranged from 4% to 23% (3, 5, 14). The wide range of variability in terms of the reported minor complications is probably attributable to the differences in definitions between the trials, such as the threshold for reporting a groin ooze or a very small hematoma. In this study, slow oozing of blood from the access site that required no additional treatment was excluded as a minor complication. That is why our study reported such a remarkably low incidence of minor complications as compared with prior studies. However, when using the same definitions for both the Angio-Seal and the Closer S groups, we found no differences between the two devices in this regard.
Closure devices were safely used in patients who had undergone previous femoral arterial punctures, including those patients who had undergone the deployment of previous closure devices. There were no statistically significant differences between the first and repeated femoral arterial puncture subgroups, in both the Angio-Seal and Closer S groups.
In addition to complications, the primary disadvantage of closure devices is the local discomfort during or after the procedure. However, our patients did not complain of discomfort for at least 24 hours after the procedure, and the restriction on ambulation and discomfort during the use of standard compressive therapy tends to compensate for the discomfort inherent to this procedure. Finally, the closure devices tend to increase the cost of the catheterization procedure. Whether this cost will be offset by reduced complications requires more specific study.
The limitation of this study was that there was a difference of 246 cases between the two groups, although this was randomized. In our center, 322 Angio-Seal devices were used at the beginning of the study before the Closer S device was used. Since interventional radiologists and neuroradiologists tried to use both devices to avoid a bias for choosing between the two devices for a patient, the difference in the number of cases did exist.
In conclusion, the use of Angio-Seal was found to be more effective than that of the Closer S for the hemostasis of femoral access sites. Angio-Seal is easier to use and it requires a shorter learning time than does the Closer S. There were no differences found with regard to the rates of complications between the Angio-Seal and the Closer S groups.
Figures and Tables
Fig. 2
Right common femoral artery occlusion (white arrow) in a 63-year-old woman receiving Angio-Seal at one month after transarterial chemoembolization.
A. Right femoral arteriography.
B. Collateral circulation has already developed.
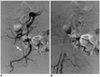
Fig. 3
A pseudoaneurysm (arrow) in a 57-year-old man who received the Closer S at 1 week after TACE.
A. Right common femoral CT angiography.
B. Right common femoral angiography.
C. A pseudoaneurysm was treated by thrombin injection.
References
1. Kussmaul WG III, Buchibinder M, Whitlow PL, Aker UT, Heuser RR, King SB, et al. Rapid arterial hemostasis and decreased access site complications after cardiac catheterization and angioplasty: results of randomized trial of novel hemostatic device. J Am Coll Cardiol. 1995. 26:1685–1692.
2. Sanborn TA, Gibbs HH, Brinker JA, Knopf WD, Kosinski EJ, Roubin GS. A multicenter randomized trial comparing a percutaneous collagen hemostasis device with conventional manual compression after diagnostic angiography and angioplasty. J Am Coll Cardiol. 1993. 22:1273–1279.
3. Cremonesi A, Castriota F, Tarantino F, Troiani E, Ricci E, El Jamal B, et al. Femoral arterial hemostasis using the Angio-Seal system after coronary and vascular percutaneous angioplasty and stenting. J Invas Cardiol. 1998. 10:464–469.
4. Kapadia SR, Raymond R, Knopf W, Jenkins S, Chapekis A, Ansel G, et al. The 6 Fr Angio-Seal arterial closure devise: results from a multicenter prospective registry. J Am Cardiol. 2001. 87:789–791.
5. Silber S, Bjorvik A, Muhling H, Rosch A. Usefulness of collagen plugging with VasoSeal after PTCA as compared to manual compression with identical sheath dwell times. Cathet Cardiovasc Diagn. 1998. 43:421–427.
6. Schrader R, Steinbacher S, Burger W, Kadel C, Vallbracht C, Kaltenbach M. Collagen application for sealing of arterial puncture sites in comparison to pressure dressing: a randomized trial. Cathet Cardiovasc Diagn. 1992. 27:298–302.
7. Carere RG, Webb JG, Ahmed T, Dodeck AA. Initial experience using Prostar: a new device for percutaneous suture-mediated closure of arterial puncture sites. Cathet Cardiovasc Diagn. 1996. 37:367–372.
8. Chamberlin JR, Lardi AB, McKeever LS, Wang MH, Ramadurai G, Grunenwald P, et al. Use of vascular sealing device (VasoSeal and Perclose) versus assisted manual compression (Femostop) in transcatheter coronary interventions requiring abciximab (ReoPro). Cathet Cardiovasc Intervent. 1999. 47:143–147.
9. Gerckens U, Cattelaens N, Lampe EG, Grube E. Management of arterial puncture site after catheterization procedures: evaluating a suture-mediated closure device. Am J Cardiol. 1998. 83:1658–1663.
10. Shrake KL. Comparison of major complication rates associated with four methods of arterial closure. Am J Cardiol. 2000. 85:1024–1025.
11. Baim DS, Knopf WD, Hinohara T, Schwarten DE, Schatz RA, Pinkerton CA, et al. Suture-mediated closure of the femoral access site after cardiac catheterizations: results of the Suture to Ambulate and Discharge (STAND I and STAND II) trials. Am J Cardiol. 2000. 85:864–869.
12. Sievert H, Wander T, Mussing S, Bolscher S. The Perclose device for hemostasis after antegrade puncture of the femoral artery: a randomized trial. Am J Cardiol. 1998. 82:Suppl 7A. 34S–35S.
13. Popma JJ, Satler LF, Pichard AD, Kent KM, Campbell A, Chuang YC, et al. Vascular complications after balloon and new device angioplasty. Circulation. 1993. 88(pt 1):1569–1578.
14. Muller DW, Shamir KJ, Ellis SG, Topol EJ. Peripheral vascular complications after conventional and complex percutaneous coronary interventional procedures. Am J Cardiol. 1992. 69:63–68.
15. Waksman R, King SB, Douglas JS, Shen Y, Ewing H, Mueller L, et al. Predictors of groin complications after balloon and new device coronary intervention. Am J Cardiol. 1995. 75:886–889.
16. Oweida SW, Roubin GS, Smith RB, Salam AA. Postcatheterization vascular complications associated with percutaneous transluminal coronary angioplasty. J Vasc Surg. 1990. 12:310–315.
17. Skillman JJ, Ducksoo K, Baim DS. Vascular complications of percutaneous femoral cardiac interventions: incidence and operative repair. Arch Surg. 1988. 123:1207–1212.
18. Babu SC, Piccorelli GO, Shah PM, Stein JH, Clauss RH. Incidence and results of arterial complications among 16,350 patients undergoing cardiac catheterization. J Vasc Surg. 1989. 10:113–116.
19. Kaufmann J, Moglia R, Lacy C, Dinerstein C, Moreyra A. Peripheral vascular complications from percutaneous transluminal coronary angioplasty: a comparison with transfemoral cardiac catheterization. Am J Med Sci. 1989. 297:22–25.
20. EPIC Investigators. Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. N Engl J Med. 1994. 330:956–961.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download