Abstract
Objective
We wanted to evaluate the feasibility and efficacy of using a dexamethasone (DM)-eluting nitinol stent to inhibit the pseudointimal hyperplasia following stent placement in the transjugular intrahepatic portosystemic shunt tract (TIPS) of a swine.
Materials and Methods
Fifteen stents were constructed using 0.15 mm-thick nitinol wire; they were 60 mm in length and 10 mm in diameter. The metallic stents were then classified into three types; type 1 and 2 was coated with the mixture of 12% and 20%, respectively, of DM solution and polyurethane (PU), while type 3 was a bare stent that was used for control study. In fifteen swine, each type of stent was implanted in the TIPS tract of 5 swine, and each animal was sacrificed 2 weeks after TIPS creation. The proliferation of the pseudointima was evaluated both on follow-up portogram and pathologic examination.
Results
One TIPS case, using the type 1 stent, and two TIPS cases, using the type 2 stent, maintained their luminal patency while the others were all occluded. On the histopathologic analysis, the mean of the maximum pseudointimal hyperplasia was expressed as the percentage of the stent radius that was patent, and these values were 51.2%, 50% and 76% for the type 1, 2, and 3 stents, respectively.
Transjugular intrahepatic portosystemic shunt (TIPS) is an effective and commonly used method for not only lowering portal pressure, but also for controlling acute variceal bleeding that is retractable to standard medical treatment for the patients with portal hypertension. However, the one-year primary patency rates after TIPS creation are 23-50%, and the long-term patency of TIPS has been disappointing because the rate for the late parenchymal tract abnormalities such as restenosis and occlusion was 75%, and the cumulative patency rate was 5% at 2 years (1-3). TIPS is often associated with the development of pseudointimal hyperplasia within the stented lumen of the hepatic parenchymal tract.
Restenosis after stent implantation is also one of the problems in vessels, and it is mainly characterized by an inflammatory response to procedural injury and an intense fibrocellular response (4). To overcome this problem, drug-eluting stents with anti-inflammatory and immunosuppressive effects have been developed, and their clinical applications have been tried in such vessels as the coronary artery. Some studies have recently reported that the dexamethasone (DM)-eluting stent was effective to reduce the myointimal proliferation in vessels. The percentage of diameter stenosis was lower in the unstable angina pectoris patients who underwent placement of DM-eluting stents compared to the stable patients (26.86% +/- 14 vs. 38.40% +/- 16%, respectively p < 0.02) (5, 6).
However, the implantation of drug-eluting stent for local drug delivery in TIPS has not been reported on in the medical literature. We hypothesized that the DM released from a DM-eluting stent would inhibit pseudointimal hyperplasia in the TIPS tract and improve vessel patency. We made the DM-eluting nitinol stents and we performed an experimental study with using them in a swine model. The purpose of this study was to evaluate the feasibility and efficacy of the DM-eluting nitinol stent for reducing the pseudointimal hyperplasia in the TIPS tract of a swine model with induced-portal hypertension.
We constructed both the stents and the introducer set in our research laboratory. The metallic stent was knitted from a single thread of 0.15 mm nitinol wire filament (Euroflex, Germany) into a tubular configuration and in an interlocking diamond-shaped pattern with 6 bent points on the upper and lower end portions. All the stents were 10 mm in diameter and 60 mm in length when they were fully expanded.
The DM-eluting stent was made by dipping a metallic stent in a solution of DM and polyurethane (PU). The metallic stents were classified into three types. Type 1 and 2 stents were coated with 12% and 20%, respectively, of the DM solution mixed with PU, while the type 3 stent was a bare nitinol stent that was used for the control study.
To evaluate the amount of DM that was released from the membrane of the DM/PU mixture that included 12% DM, a stent with a 1 cm2 membrane was soaked in a tube with 0.1 M neutral phosphate buffer solution, and the bottle was placed in a shaking incubator. The temperature of the incubator was set to 37℃, and the bottle was continuously rotated. The buffer solution was exchanged every 24 hours, and the amount of DM in the buffer solution was measured by performing high performance liquid chromatography (Rainin Instrument Co., Wolburn, MA) at one month. The wavelength to detect the DM was 254 nm, which was the peak absorption point of DM on the preliminary test.
All the experimental procedures were performed under the National Institute of Health guidelines for the humane handling of animals and our study was approved by the committee for animal research at our institution.
Transjugular intrahepatic portosystemic shunt was performed in 15 domestic pigs that had an initial weight of 15-20 kg each. The authors performed the procedure by using the techniques described in the previous reports (7, 8). Anesthesia was induced in each swine with an intramuscular injection of ketamine hydrochloride (Yuhan Corporation, Seoul, Korea), and the anesthesia was maintained by intravenous injections whenever needed. Under fluoroscopic guidance, the right internal jugular vein was cannulated while iodinated contrast material was injected through the ear vein, and a 0.035-inch guide wire (Radiofocus, Terumo, Tokyo, Japan) was then inserted. The tract was gradually dilated up to 9 Fr with a dilator and a 9-F Teflon sheath (Transjugular Liver Access Set; Cook, Bloomington, IN) was coaxially loaded over the guide-wire and advanced caudally over the guide wire into the inferior vena cava and hepatic vein. The right hepatic vein was selected for using a 5 Fr cobra catheter (Cook), and a hepatic venogram and wedge portogram were taken for visualization of the portal vein. For the puncture, a sheathed 16-gauge Colapinto needle (Cook) was placed into the right hepatic vein, and the portal vein was punctured via the intrahepatic route. After the confirmation of the portal vein by the aspiration of venous blood and the injection of contrast media, a guide wire was advanced into the portal vein and the needle was removed. After the advancement of a 5 Fr Cobra catheter into the portal vein, a direct portogram was taken and the portal pressure was measured (Fig. 1A). To induce portal hypertension, a microcatheter (Progreat, Terumo, Tokyo, Japan) was inserted into the portal vein through a cobra catheter, and an intraportal injection of a mixture of N-butyl-2-cyanoacrylate and lipiodol (1:3) was done. When the migration of the mixture from the catheter tip to the peripheral portion of the portal vein was sluggish, injection of the mixture was stopped; the total amount of the mixture was approximately 1-2 ml. After embolization of the portal vein, the occluded portal perfusion on the follow-up portogram was confirmed, as well as confirming the increased portal pressure (Fig. 1B). The parenchymal tract was dilated with a balloon catheter (10 mm in diameter, 4 cm in length), and a nitinol stent was placed in the tract. The final portogram was taken and the portal pressure was again measured (Fig. 1C). Each type of stent was implanted in the TIPS tract of 5 swine.
After two weeks, the animals were sedated with the same method that was used in the TIPS procedure. To obtain the follow-up shunt venogram, the portal vein was selected by the same method as was used in the TIPS procedure for the swine with a patent tract (Fig. 2A). Meanwhile, in the swine with the completely occluded TIPS tract, a direct portogram was taken by the following method after performing hepatic venogram. The skin of abdomen was incised about 10 to 15 cm in length at the midline from the epigastrium. After exposing the small bowel, the tributary of the superior mesenteric vein was punctured and the direct portogram was taken each second after the hand injection of 10 ml of contrast media (Fig. 2B). Each animal was sacrificed with an intravenous injection of sodium pentobarbital solution (Hanlim Pharm. CO., LTD, Seoul, Korea) for the histologic analysis of the tissue around the TIPS. The liver, the supra-hepatic and infra-hepatic inferior vena cava and the extrahepatic portal vein were excised en bloc. The cross sections of the TIPS tract were obtained, and the specimens were evaluated by staining with hematoxin-eosin and Masson-trichrome. The maximal distance of the pseudointimal hyperplasia from the stent was measured for each animal on the histopathologic examination. The degree of the maximal pseudointimal hyperplasia was calculated by dividing the stent radius by the thickness of the pseudointimal hyperplasia without inclusion of any thrombus (Fig. 3).
The mean increased weight of the stent coated with the DM and PU mixture was 22.3 mg and 25.1 mg for the type 1 and 2 stents, respectively. The mean weight of the DM, as calculated from the concentration of DM in the solution of the DM and PU mixture, was 2.7 mg and 5.0 mg for the type 1 and 2 stents, respectively.
The in-vitro examination using high performance liquid chromatography showed that the amount of DM released for the first from the membrane of the DM/PU mixture was 25% of the loaded dose. The accumulated amounts of the released DM for the first three days and two weeks were 50% and 70%, respectively. The total amount of released DM at one month was 80% (Fig. 4).
Transjugular intrahepatic portosystemic shunt procedures were technically successful in all the swine; all the stents were deployed accurately without any complication in all the swine. Although the stents partially expanded in the intraparenchymal portion of all swine, an additional balloon dilation was not done because the blood flow to the hepatic vein was improved without disturbance, and the portal pressure was decreased below the initial pressure.
The mean pressure in the portal vein was 23.44 cmH2O and 37.94 cmH2O before and after the injection of N-butyl-2-cyanoacrylate, respectively, and then this immediately dropped back to 19.72 cmH2O following TIPS.
On the follow-up portogram taken two weeks after the stent placement, one case of the 5 swine with the type 1 stent and two cases of the 5 swine with the type 2 stent displayed luminal patency despite of the focal narrowing of the lumen in the intraparenchymal portion (Fig. 2A). The remaining cases using type 1 and 2 stents and all the cases of the 5 swine with the type 3 stent showed completely occluded lumens of their stents (Fig. 2B).
On the histopathologic analysis, the mean of the maximal pseudointimal hyperplasia were 51.2%, 50% and 76% for the type 1, 2, and 3 stents. The microscopic findings showed a relatively thin and uniform pseudointimal hyperplasia within the patent TIPS tract in the type 1 and 2 stents, and there was complete occlusion of the TIPS tract by pseuointimal hyperplasia in the type 3 stent (Figs. 5A, B). The psuedointimal hyperplasia consisted of myofibroblasts and an extracellular matrix that had grown through the stent wires, and inflammatory cells had infiltrated around the stent wires.
Although TIPS is an effective method for reducing the portal venous pressure in the patient with variceal bleeding or intractable ascietes due to portal hypertension, a high rate of restenosis and occlusion of the tract are the critical drawbacks of this technique (1-3). The important mechanisms of restenosis or occlusion of the shunt, as reported in the previous reports, were acute thrombosis, chronic inflammatory reaction, pseudointimal hyperplasia and inhibition of the endothelialization process associated with bile duct injury and bile extravasation within the stented lumen of the hepatic parenchymal tract (9-12). The layers of pseudointimal hyperplasia consist of mesenchymal cells and collagen, and this is covered by a layer endothelial cells. Various techniques and prosthesis were developed to reduce the rate of restenosis and to increase primary patency. Some experimental studies that have used stentgrafts covered with polyethylene terephthalate, polytetrafluoroethylene, dacron, silicone or polycarbonate urethane were performed in the TIPS tract (13-17). However, the placement of a stent-graft in the TIPS tract did not prevent the pseudointimal hyperplasia and it only provided patency equal to that of the bare stent due to the stent's early occlusion or because of late shunt malfunction in selected cases. Lessie et al. reported on intraluminal irradiation for TIPS stenosis in a swine model, but the irradiation of the TIPS did not significantly improve patency or reduce the degree of pseudointimal hyperplasia (18).
Many investigations have been performed to prevent restenosis after stent implantation in the vessel. To reduce the rate of restenosis that's due to an inflammatory response to procedural injury and then the intense fibrocellular response, drug-eluting stents with anti-inflammatory and immunosuppressive effects have been developed and clinical applications have been tried in various vessels; DM is one of the drugs that has been used. DM is a glucocorticoid with potent anti-inflammatory, anti-fibrotic, anti-thrombotic and anti-proliferative properties, and it interferes with macrophages and reduces the level of growth factors and cytokines. In vessels, the DM acts to prevent both adherence and aggregation of granulocytes to the endothelial cells, and the intimal hyperplasia mediated by the leukocytes is suppressed (19). DM demonstrated the ability to suppress myointimal hyperplasia in an in vitro experiment using a culture of smooth muscle cell (20-22). However, previous clinical studies have reported that systemic intravenous, intramuscular or peroral administrations of corticosteroids were not effective treatments for reducing the restenosis after percutaneous transluminal angioplasty (23, 24). As for the results of using DM-eluting stents for placement in vessels, some reports have suggested the beneficial effects of DM-eluting stents on the migration and proliferation of the smooth muscle cells of the vessels, and other reports have suggested that DM-eluting stent could not reduce neointimal thickening and thrombotic occlusion within a stent (5, 6, 25-27). Strecker et al have reported that DM-coated stents reduced the neointimal hyperplasia in the femoral arteries of dogs (25). Yoon et al. performed the experiment with placement of a DM-coated stent in the canine aorta and inferior vena cava, and they reported that the stent was effective for decreasing the neointimal formation. Yoon and coworkers proved the effectiveness of DM-eluting stent in veins (27). Contrary to obtaining positive results, Lincoff et al. and Muller et al. reported their negative results of placing DM-eluting stent in vessels. In those reports, the DM-eluting stent was well tolerated within the coronary vessel, but local delivery of DM by the intravascular DM-eluting stent did not decrease the neoinitmal hyperplasia in porcine coronary and carotid arteries (5, 26).
In this study, DM was effective to inhibit the pseudointimal hyperplasia in the TIPS tract. Contrary to histopathologic finding of the control stent, i.e., complete occlusion of the TIPS tract by being filled with numerous spindle-shaped myofibroblasts and a collagen matrix, both types of DM-eluting stents having 12% and 20% DM concentrations suppressed the pseuointimal hyperplasia. The findings of a thinner pseudointimal layer than the control stent and the relatively uniform pattern of pseudointimal hyperplasia in DM-eluting stents may be due to the effects of the DM. We suspected that DM-eluting stent in the TIPS tract had a mechanism similar to that seen for the inhibition of myointimal hyperplasia in the arteries with DM eluting stents (10). However, despite the inhibition of the psedointimal hyperplasia by the DM, the occlusion of TIPS did occur, and the vessel patency was not prolonged in the TIPS with using the DM-eluting stents. These results suggested that the anti-proliferative effect of DM, which was eluded from stent alone, was sufficient to reduce pseudointimal hyperplasia in the TIPS tract, but many other factors might have also promoted the stenosis and occlusion of the TIPS tract. In this study, the formation of thrombus in the TIPS tract was observed on examination of the gross specimen, and we believed that thrombosis was an important factor for the high rate of TIPS occlusion in the swine model with using the DM-eluting stent. The thrombosis we saw in the TIPS was also suggested to be an important cause of occlusion in the swine model using intraluminal irradiation for TIPS stenosis because the difference between the patent and occluded TIPS was the presence and/or absence of thrombus within the lumen (18). Although there are some controversies, the transection of major bile ducts that occurs during the formation of the TIPS tract and the leakage of bile from the injured ducts are both related to the thrombosis observed in the TIPS tract, and especially for the rapid and early thrombosis (10, 14, 28, 29). The proliferation of the bile duct within the tissue lined stenotic portion of the TIPS and the incorporation of bile within thrombi are evidence for the relation between bile leakage and thrombosis in the TIPS tract. Although the relationship between thrombosis and bile leakage was not proven in this experiment, we suggest that the thrombogenic effect was too strong to be suppressed by the anti-thrombotic effect of the DM released from the DM-eluting stents, and the drug effect was insufficient to prevent thrombosis within the TIPS tract. Any difference of the anti-thrombotic effect between the 12% and 20% DM-eluting stents was not observed because complete occlusions on the follow-up portograms were noted in both types of stents. However, further studies are needed to investigate whether the combined use of anti-thrombotic agents can reduce thrombosis and solve this problem, and so improve the patency rate for the TIPS procedure.
The concentration of DM in solution was decided upon based on results of our preliminary study with taking into consideration the physical properties of the stent and the solution. The highest concentration of DM that was achievable in solution for coating without changing the physical properties of the nitinol stent was 12%, and the radial force of the stent coated by the solution with a higher concentration of DM showed a decrease in strength. The highest possible concentration for coating the stent was 20% because of the viscosity of the solution. Although Yoon et al reported coating the nitinol stents with 25% DM-PU solution, twenty percentage was the highest concentration we could do with our method of coating (27). Since there was no significant difference in the thickness of pseudointimal hyperplasia and anti-thrombotic effect between the 12% and 20% DM eluting stents, the placement of a 12% DM stent is recommended rather than placement of a 20% DM stent to inhibit pseudointimal hyperplasia in the TIPS tract. When comparing both types of stents, the 20% DM stent will not provide any benefit over the 12% DM stent for the preservation of the physical properties of the nitinol stent since the increased viscosity of the 20% DM solution makes coating the stent extremely difficult. The 20% DN stent will merely increase the concentration of DM in the serum.
The reason why the animals were followed-up for only for two weeks after the creation of TIPS was that in the swine model, all the TIPS tracts were occluded after two week. Although swine models are widely used because TIPS can be performed with the same techniques and devices used in human and the healing responses after surgery are similar to those of human, the animals rapidly increase in weight and size, and they can be difficult to handle. In another study, stenosis and occlusion progressed within 2-4 weeks of TIPS formation in swine (14). In this study, all the TIPS tracts with using the control stent were completely occluded by myofibroblasts, extracellular matrix and inflammatory cells by two weeks following TIPS; so, a two week follow-up period might be sufficient.
In conclusion, although the patency of the TIPS tract did not improve because of the partial occlusion by thrombi, the DM-eluting stent showed a tendency to reduce the development of pseudointimal hyperplasia in the TIPS tract of a swine model with induced portal hypertension.
Figures and Tables
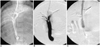 | Fig. 1Portograms during stent placement.
A. Under the fluoroscopic guidance, the portal vein was punctured via the right jugular vein, and the portogram was obtained.
B. Portogram that was obtained after inducing portal hypertension by an injection of a mixture of N-butyl-2-cyanoacrylate and lipiodol in the portal vein, and it shows the occlusion of the portal vein.
C. Portogram taken after the placement of a stent in the transjugular intrahepatic portosystemic shunt tract shows the good flow to the
hepatic vein.
|
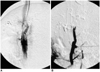 | Fig. 2Follow-up portograms taken two weeks after transjugular intrahepatic portosystemic shunt.
A. Portogram performed via the hepatic vein in the DM-releasing stent group shows a partial focal stenosis.
B. Direct portogram by puncture of a tributary of the superior mesenteric vein shows the complete occlusion in the control group.
|
 | Fig. 4In-vitro evaluation of the dexamethasone released from a membrane made from a 12% dexamethasone concentration in a dexamethasone-polyurethane solution. |
 | Fig. 5Microcopic specimen (×10) stained with H-E in a dexamethasone-releasing stent.
A. Microscopic section in the dexamethasone-releasing stent group shows a relatively thin and uniform pseudointimal hyperplasia in the patent transjugular intrahepatic portosystemic shunt tract.
B. Microscopic section from the control stent group shows the marked ingrowth of fibrotic tissue within the lumen of the stent and complete occlusion of the transjugular intrahepatic portosystemic shunt tract.
|
References
1. Zhuang ZW, Bettmann MA, Teng GJ, Jeffery RF, Langdon DR. Long-term results in patients with TIPS: an outcome study. J Vasc Interv Radiol. 2001. 12:suppl. S9–S10.
2. Haskal ZJ, Pentecost MJ, Soulen MC, Shlansky-Goldberg RD, Baum RA, Cope C. Transjugular intrahepatic portosystemic shunt stenosis and revision: early and midterm results. AJR Am J Roentgenol. 1994. 163:439–444.
3. Saxon RS, Ross PL, Mendel-Hartvig J, Barton RE, Benner K, Flora K, et al. Transjugular intrahepatic portosystemic shunt patency and the importance of stenosis location in the development of recurrent symptoms. Radiology. 1998. 207:683–693.
4. Duda SH, Poerner TC, Wiesinger B, Rundback JH, Tepe G, Wiskirchen J, et al. Drug-eluting stents: potential applications for peripheral arterial occlusive disease. J Vasc Interv Radiol. 2003. 14:291–301.
5. Lincoff AM, Frust JG, Ellis SG, Tuch RJ, Topol EJ. Sustained local delivery of dexamethasone by a novel intravascular eluting stent to prevent restenosis in the porcine coronary injury model. J Am Coll Cardiol. 1997. 29:808–816.
6. Liu X, Huang Y, Hanet C, Vandormael M, Legrand V, Dens J, et al. Study of antirestenosis with the BiodivYsio dexamethasoneeluting stent (STRIDE): a first-in-human multicenter pilot trial. Catheter Cardiovasc Interv. 2003. 60:172–178.
7. Haskal ZJ, Davis A, McAllister A, Furth EE. PTFE-encapsulated endovascular stent-graft for transjugular intrahepatic portosystemic shunts: experimental evaluation. Radiology. 1997. 205:682–688.
8. Kichikawa K, Saxon RR, Nishimine K, Nishida N, Uchida BT. Experimental TIPS with spiral Z-stents in swine with and without induced portal hypertension. Cardiovasc Intervent Radiol. 1997. 20:197–203.
9. Teng GJ, Bettmann MA, Hoopes PJ, Wagner RJ, Park BH, Yang L, et al. Transjugular intrahepatic portosystemic shunt: effect of bile leak on smooth muscle cell proliferation. Radiology. 1998. 208:799–805.
10. LaBerge JM, Ferrell LD, Ring EJ, Gordon RL. Histopathologic study of stenotic and occluded transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 1993. 4:779–786.
11. Palmaz JC, Sibbitt RR, Reuter SR, Garcia F, Tio FO. Expandable intrahepatic portacaval shunt stents: early experience in the dog. AJR Am J Roentgenol. 1985. 145:821–825.
12. Terayama N, Matsui O, Kadoya M, Yoshikawa J, Gabata T, Miyayama S, et al. Transjugular intrahepatic portosystemic shunt: histologic and immunohistochemical study of autopsy cases. Cardiovasc Intervent Radiol. 1997. 20:457–461.
13. Haskal ZJ, Brennecke LJ. Porous and nonporous polycarbonate urethane stent-grafts for TIPS formation: biologic responses. J Vasc Interv Radiol. 1999. 10:1255–1263.
14. Haskal ZJ, Brennecke LH. Transjugular intrahepatic portosystemic shunts formed with polyethylene terephthalate-covered stents: experimental evaluation in pigs. Radiology. 1999. 213:853–859.
15. Nishimine K, Saxon RR, Kichikawa K, Mendel-Hartvig J, Timmermans HA, Shim HJ, et al. Improved transjugular intrahepatic portosystemic shunt patency with PTFE-covered stent-grafts: experimental results in swine. Radiology. 1995. 196:341–347.
16. Otal P, Rousseau H, Vinel JP, Ducoin H, Hassissene S, Joffre F. High occlusion rate in experimental transjugular intrahepatic portosystemic shunt created with a Dacron-covered nitinol stent. J Vasc Interv Radiol. 1999. 10:183–188.
17. Tanihata H, Saxon RR, Kubota Y, Pavcnik D, Uchida BT, Rosch J, et al. Transjugular intrahepatic portosystemic shunt with silicone-covered Wallstents: results in a swine model. Radiology. 1997. 205:181–184.
18. Lessie T, Yoon HC, Nelson HA, Fillmore DJ, Baldwin GN, Miller FJ. Intraluminal irradiation for TIPS stenosis: preliminary results in a swine model. J Vasc Interv Radiol. 1999. 10:899–906.
19. Oseas RS, Allen J, Yang HH, Baehner RL, Boxer LA. Mechanism of dexamethasone inhibition of chemotactic factor induced granulocyte aggregation. Blood. 1982. 59:265–269.
20. Chervu A, Moore WS, Quinones-Baldrich WJ, Henderson T. Efficacy of corticosteroids in suppression of intimal hyperplasia. J Vasc Surg. 1989. 10:192–134.
21. Colburn MD, Moore WS, Gelabert HA, Quinones-Baldrich WJ. Dose responsive suppression of myointimal hyperplasia by dexamethasone. J Vasc Surg. 1992. 15:510–518.
22. Voisard R, Seitzer U, Baur R, Dartsch PC, Osterhues H, Hoher M, et al. Corticosteroid agents inhibit proliferation of smooth muscle cells from human atherosclerotic arteries in vitro. Int J Cardiol. 1994. 43:257–267.
23. Stone GW, Rutherford BD, McConahay DR, Johnson WL, Giorgi LV, Ligon RW, et al. A randomized trial of corticosteroids for the prevention of restenosis in 102 patients undergoing repeat coronary angioplasty. Cathet Cardiovasc Diagn. 1989. 18:227–231.
24. Pepine CJ, Hirshfeld JW, Macdonald RG, Henderson MA, Bass TA, Goldberg S, et al. A controlled trial of corticosteroids to prevent restenosis after coronary angioplasty. M-HEART Group. Circulation. 1990. 81:1753–1176.
25. Strecker EP, Gabelmann A, Boos I, Lucas C, Xu Z, Haberstroh J, et al. Effect on intimal hyperplasia of dexamethasone released from coated metal stents compared with non-coated stents in canine femoral arteries. Cardiovasc Intervent Radiol. 1998. 21:487–496.
26. Muller DW, Golomb G, Gordon D, Levy RJ. Site-specific dexamethasone delivery for the prevention of neointimal thickening after vascular stent implantation. Coron Artery Dis. 1994. 5:435–442.
27. Yoon HK, Park KS, Kang SG, Park SS, Kim TH, Sung KB, et al. Influence of dexamethasone-coated Nitinol stent on neointimal formation in the canine great vessel model. J Korean Radiol Soc. 2001. 44:433–440.
28. Jalan R, Harrison DJ, Redhead DN, Hayes PC. Transjugular intrahepatic portosystemic stent-shunt (TIPSS) occlusion and the role of biliary venous fistulae. J Hepatol. 1996. 24:169–176.
29. Saxon RR, Mendel-Hartvig J, Corless CL, Rabkin J, Uchida BT, Nishimine K, et al. Bile duct injury as a major cause of stenosis and occlusion in transjugular intrahepatic portosystemic shunts: comparative histopathologic analysis in humans and swine. J Vasc Interv Radiol. 1996. 7:487–497.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download