Abstract
Objective
We wanted to clarify the relationship between the visibility of air cysts on CT images, the CT slice thickness and the size of the air cysts, with contact radiographs as the gold standard, for the accurate evaluation of honeycomb cysts.
Materials and Methods
An inflated and fixed autopsied lung having idiopathic interstitial pneumonia was evaluated. The corresponding air cysts were identified on the contact radiographs of a 0.5 mm-thick-section specimen and also on the CT images of three different kinds of section thickness: 0.5, 1.0 and 2.5 mm. The maximal diameters of the air cysts were measured under a stereomicroscope.
Results
A total of 341 air cysts were identified on the contact radiograph, and they were then evaluated. Sixty-six percent of air cysts 1 to 2 mm in diameter were detected by 0.5 mm slice thickness CT, while only 34% and 8% were detected by 1.0 and 2.5 mm slice thickness CT, respectively. Only 28% and 22% of air cysts less than 1 mm in diameter were detected by 0.5 and 1.0 mm slice thickness CT, respectively. CT with a 2.5 mm slice thickness could not demonstrate air cysts less than 1 mm in diameter.
According to the consensus classification by the ATS (American Thoracic Society) and the ERS (European Respiratory Society), idiopathic pulmonary fibrosis (IPF) is defined as a distinctive type of chronic fibrosing interstitial pneumonia of unknown etiology, and it is associated with a pathological pattern of usual interstitial pneumonia (UIP) on the surgical lung biopsy specimen (1). The consensus statement stressed that the primary role of high-resolution CT (HRCT) is to separate patients with UIP lesions from those patients with non-UIP lesions or from those patients with less specific findings that are associated with other idiopathic interstitial pneumonias. The presence of clinical symptoms and the HRCT features of UIP is sufficiently distinct to allow a confident diagnosis and thus, to eliminate the need for a surgical lung biopsy specimen.
The characteristic HRCT findings for UIP are a bilateral, predominantly basal and subpleural reticular pattern that is associated with subpleural cysts (honeycombing) and/or traction bronchiectasis. As honeycombing is a rare finding in non-UIP lesions, it is very important to differentiate IPF from the other interstitial pneumonias. In addition, the evaluation of honeycombing is important for the management of patients with IPF because it generally means the patient will have a poor response to treatment (1-3). On the HRCT images, honeycombing is recognized as thick walled air cysts, and this finding is particularly evident in the subpleural region (4-6). Some investigators have reported that on HRCT images with a 1.5-2 mm slice thickness, the diameter of visible air cysts ranges from 0.3 to 1.0 cm, but these cysts may be up to 2.5 cm in size (7). Other investigators have reported the size of the cysts ranges from 2 to 20 mm (8). Recent CT scanner developments, which enable the use of less than 1 mm collimation, have allowed a more precise evaluation of honeycombing by reducing the partial volume effect and improving the spatial resolution.
Although the utility of CT for evaluating honeycombing has been widely accepted, there have been no precise reports focusing on the relationship between the visibility of air cysts on CT images, the slice thickness of CT and the size of the air cysts. In addition, it is also important to test the ability of newly developed sub-millimeter CT for demonstrating honeycombing in those patients suspected of having IPF.
The purpose of this study is to evaluate the visibility of air cysts on various slice thickness CT images, including 0.5, 1.0, and 2.5 cm slice thicknesses, for the accurate evaluation of honeycombing. We did this by using an inflated and fixed IPF lung specimen with the contact radiograph as the gold standard.
We studied a lung that was excised at autopsy from an 85-year-old woman who had IPF. The lung was inflated and fixed using a standard method that was previously reported (9). The lung was distended through a main bronchus with a fixative fluid containing polyethylene glycol 400, 95% ethyl alcohol, 40% formalin and plain water mixed in proportions of 10:5:2:3, and the lung was next immersed in the fixative for two days. The fixed lung was then air dried and cut into 2 cm thick transaxial slices.
The CT scanner was a SOMATOM Plus4 Volume Zoom (Siemens, Erlangen, Germany). The specimen slices were scanned with the following technical factors: an axial mode, 120 kV, 150 mAs, 0.5, 1, and 2.5 mm collimation/slice thickness, 70 mm field of view (FOV) and a Bf60 (high spatial resolution) reconstruction algorithm. All the images were observed and photographed at a window of 1500 HU and a level of -650 HU.
After the CT examination, a rectangular block measuring 7×7 cm was excised from a 2 cm thick specimen. This block was then cut into contiguous 0.5 mm thick slices as near to the plane of the CT images as possible with the use of a microslicer (DTW 1000 W; Dosaka EM, Kyoto, Japan). The contact radiographs of these slices were obtained with a fine-grain film (X-OMAT-TL film, Eastman-Kodak, Rochester, NY) at 15 kVp, 90 mAs and a 65 cm tube-film distance.
Air cysts having an identifiable wall were detected on the 0.5 mm thick specimen and its contact radiograph (Fig. 1A). This slice corresponded to the mid-portion of the 2 cm thick specimen. This procedure was done by manual tracing each cyst on the translucent paper that was placed over the contact radiograph. The maximal diameters of the air cysts were subsequently measured by using a micrometer caliper under stereomicroscopic observation. These procedures were performed by one experienced chest radiologist (Y.N.). The continuity of the airway structure in the serial slices was helpful when the observer had difficulty in differentiating air cysts from airways that were of the same size.
A CT image was chosen from each image series of every slice thickness that corresponded to the mid-portion within the specimen (Figs. 1B-D). Air cysts with identifiable walls were detected on the CT images in the same manner as on the contact radiograph, and this was done by two experienced chest radiologists working in consensus (M.T., K.M.). They were kept unaware of the specimen and the contact radiograph findings. On the CT images, the air cysts were identified by visual observation as those lesions showing air densities with definable thick walls.
Finally, the identified air cysts on the contact radiograph were compared with those cysts identified on the CT images one by one. This procedure was carried out by three experienced chest radiologists (Y.N., M.T., K.M.) working in consensus. From this comparison, we attempted to clarify the relationship among the visibility of air cysts on the CT images, the CT slice thickness (0.5, 1.0, 2.5 mm) and the size of the air cysts.
A total of 341 air cysts were identified on the contact radiographs. Figure 2 shows the frequency distribution of the maximal diameter of the air cysts detected on the contact radiographs. The smallest air cyst diameter was 0.7 mm and the largest was 13.1 mm. Most air cysts (84.5%: 288/341 air cysts) measured between 1.0 to 3.9 mm. Among them, cysts 1 to 1.9 mm in diameter were the most frequently observed (134/341, 39.3%).
Figure 3 shows the relationship among the ratio of number of the detected air cysts to the number of visible air cysts on the CT images, the size of cysts and the slice thickness. The visibility of air cysts was obtained by using the contact radiograph as the standard. With 0.5 mm slice thickness, the visibility of air cysts smaller than 1.0 mm in diameter was 28%, 1.0-1.9 mm: 66%, for 2.0-2.9 mm: 93%, 3.0-3.9 mm: 85% and larger than 4.0 mm: 100%. With 1 mm slice thickness, the visibility of air cyst smaller than 1.0 mm in diameter was 22%, 1.0-1.9 mm: 34%, 2.0-2.9 mm: 80%, 3.0-3.9 mm: 68% and larger than 4.0 mm: 100%. With using 2.5 mm slice thickness, it was difficult to detect air cysts smaller than 2.0 mm in diameter and the visibility of air cysts from 2.0 to 2.9 mm in diameter was 36%, 3.0-3.9 mm: 45%, 4.0-4.9 mm: 76%, and larger than 5.0 mm: 94%.
Honeycombing is defined as the manifestation of scarring and the architectural restructuring that follows lung injury due to a variety of causes such as UIP, collagen lung, asbestosis, sarcoidosis, etc. It is considered as irreversible change and is sometimes referred to as "end-stage" lung disease (2). Histopathologically, honeycombing is characterized by enlarged air spaces that are lined by bronchiolar epithelium or hyperplastic alveolar pneumocytes, and the air spaces are separated by thick walls containing collagen and varying amounts of chronic inflammation. On HRCT scans, honeycombing is defined as cystic air spaces ranging from a few millimeter to several centimeter in diameter, and it is characterized by clearly definable walls that can be thick (6). The air cysts can range in size from 2 to 25 mm (7-8).
High-resolution CT with 1.5-3 mm collimation has become an integral tool for the evaluation of the patients with diffuse lung disease (10-12). Although HRCT's clinical utility for assessing honeycombing has been widely acknowledged, no precise reports have been published describing the relationship among the visibility of air cysts on CT images, the slice thickness of CT and the size of the air cysts. There has been only one paper by Noma et al. in which a precise correlation was performed between cyst size and their visibility on HRCT (13). In this study, the HRCT findings of 23 patients with interstitial pneumonia were reviewed and the findings were correlated with the biopsy specimens obtained by video-assisted thoracoscopic surgery. Two radiologists and two pathologists independently interpreted whether honeycombing was present or not on the HRCT images and in the pathological specimens, respectively. On the 23 HRCT images, honeycombing was present on five images and absent in 18 images, while it was pathologically present in 15 specimens and absent in eight specimens. The physicians mentioned that air cysts less than 2 mm in diameter in the histopathologic specimens could not be recognized as honeycombing on HRCT images taken with 2 mm collimation. It is also important to know the ability of the recently developed sub-millimeter CT images for demonstrating honeycombing as these images theoretically have superior spatial resolution over the conventional HRCT images.
In this study, the visibility of air cysts on the CT images was evaluated for variable slice thickness by using an inflated and fixed IPF lung specimen. As a result, air cysts less than 2 mm in diameter were recognized on CT images with 0.5 and 1 mm slice thickness CT images. About one third of air cysts that were even less than 1 mm in diameter were recognized with 0.5 mm collimation. The visibility of air cysts less than 4 mm in diameter on the 0.5 mm slice thickness CT images was slightly superior to that of the 1 mm slice thickness CT images. All of air cysts more than 4 mm in diameter were recognized with 0.5 and 1 mm slice thickness CT images. On 2.5 mm slice thickness CT images, the visibility of air cysts less than 5 mm in diameter was inferior to those of the 0.5 and 1 mm slice thickness CT images, but almost all of air cysts more than 5 mm in diameter were detected. According to these results, it is concluded that care must be taken to consider the CT slice thickness when evaluating honeycombing via CT.
It is especially noteworthy that CT images with a 0.5 mm slice thickness were better than the 1 mm CT images in demonstrating air cysts less than 2 mm in diameter. Sixty-six percent of air cysts less than 2 mm in diameter were detected by the 0.5 mm slice thickness CT images, while only 34% were detected by the 1.0 mm slice thickness CT images. This result shows that the newly-developed sub-millimeter CT has a significant potential for depicting the small air cysts that the previous CT images could not detect. The ATS and ERS statement stressed that the radiologist must first determine the presence or the absence of a pattern of IPF/UIP when interpreting the HRCT scans of a patient with diffuse lung disease (1). The evaluation of honeycombing with HRCT for the diagnosis of IPF is extremely important since the presence of bibasilar reticular abnormalities with minimal ground glass opacities on the HRCT images has been adopted as one of the major diagnostic criteria in the absence of a surgical lung biopsy, and current treatment is not indicated for those patients with end stage honeycomb lung. On the contrary, the underestimation of honeycombing with 2.5 mm collimation has led to the misinterpretation of ground glass opacity as an alveolitis condition that predicts physiological improvement of the patient when steroid therapy is administered.
This study has the following limitations. First, detection of the air cysts on the contact radiograph is an objective procedure and the reproducibility of our results may be limited since only one investigator performed this procedure. Second, these results cannot be directly translated to the clinical CT images as they were obtained from an in-vitro situation in which a small FOV was employed and there was no quantum noise from the chest wall or motion artifacts from respiratory movement and cardiac pulsation. Ideally, the HRCT images of interstitial pneumonia should be directly correlated with a surgical specimen, as Noma et al. did in their study (13). However, the results of our study were enough to caution radiologists that the term "honeycombing" should be carefully used when interpreting HRCT images for those patients with interstitial pneumonia. It should be noted that even with submilimeter CT, its ability to detect a honeycomb cyst is not completely satisfactory. Third, since there is a range of cyst sizes, it is very difficult to extrapolate from the data we presented on how often honeycombing may be clinically missed in a given patient if HRCT images with 0.5 mm slice thickness are used. However, our results are satisfactory for demonstrating the different potential of 0.5 mm HRCT for depicting honeycomb cysts from the conventional HRCT.
Our conclusion is that radiologists should recognize the ability and limitations of CT for depicting honeycombing in consideration of the slice thickness and size of the cysts because the detection rate of honeycombing by CT is significantly influenced by both the slice thickness and the cyst size.
Figures and Tables
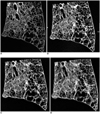
Fig. 1
A. Idiopathic pulmonary fibrosis in an 85-year-old woman. Contact radiograph of the specimen (0.5 mm thick slice). Air cysts from 1.0 to 4.0 mm in diameter were concentrated in the area of the right upper quadrant. Air cysts from 6.2 to 12.6 mm in diameter existed in the area of right lower quadrant.
B. On 0.5-mm-slice-thickness CT image cysts are clearly visudized.
C. On 1-mm-slice-thickness CT image, every air cyst was clearly described. The walls of the air cysts were more definite on the 0.5 mm slice thickness CT images than with 1 mm slice thickness CT images.
D. On 2.5-mm-slice-thickness CT image, the margins of the small air cysts became unclear and some were recognized as reticular shadow superimposed ground grass opacity. The large air cysts were the same as on the contact radiograph.
References
1. Travis WD, King TE JR, Bateman ED, et al. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002. 165:277–304.
2. Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med. 1998. 157:1301–1315.
3. King TE JR, Costabel U, Cordier J-F, et al. American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. Am J Respir Crit Care Med. 2000. 161:646–664.
4. Itoh H, Murata K, Konishi J, Nishimura K, Kitaichi M, Izumi T. Diffuse lung disease: pathologic basis for the high-resolution computed tomography findings. J Thorac Imaging. 1993. 8:176–188.
5. Nishimura K, Kitaichi M, Izumi T, Nagai S, Kanaoka M, Itoh H. Usual interstitial pneumonia: histologic correlation with high-resolution CT. Radiology. 1992. 182:337–342.
6. Webb WR, Muller NL, Naidich DP. Standardized terms for high-resolution computed tomography of the lung: a proposed glossary. J Thorac Imaging. 1993. 8:167–175.
7. Fraser RS, Muller NL, Colman N, Pare PD, editors. Terms for CT of the lungs. Diagnosis of diseases of the chest. 1999. 4th ed. Philadelphia: W.B. Saunders;xxxiii–xxxvi.
8. Muller NL, Miller RR, Webb WR, Evans KG, Ostrow DN. Fibrosing alveolitis: CT-pathologic correlation. Radiology. 1986. 160:585–588.
9. Heitzman ER. The lung: radiologic-pathologic correlations. 1984. 2nd ed. St. Louis: Mosby;4–12.
10. Nakata H, Kimoto T, Nakayama T, Kido M, Miyazaki N, Harada S. Diffuse peripheral lung disease: evaluation by high-resolution computed tomography. Radiology. 1985. 157:181–185.
11. Muller NL, Staples CA, Miller RR, Vedal S, Thurlbeck WM, Ostrow DN. Disease activity in idiopathic pulmonary fibrosis: CT and pathologic correlation. Radiology. 1987. 165:731–734.
12. Murata K, Khan A, Rojas KA, Herman PG. Optimization of computed tomography technique to demonstrate the fine structure of the lung. Invest Radiol. 1988. 23:170–175.
13. Noma S, Kubo T, Kuroda Y, et al. Definition of honeycombing on HRCT and microscopic honeycombing. Jpn J Clin Radiol. 1999. 44:73–77.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download