Abstract
Objective
We wanted to valuate the mid-term therapeutic results of percutaneous transhepatic balloon angioplasty for portal vein stenosis after liver transplantation.
Materials and Methods
From May 1996 to Feb 2005, 420 patients underwent liver transplantation. Percutaneous transhepatic angioplasty of the portal vein was attempted in six patients. The patients presented with the clinical signs and symptoms of portal venous hypertension or they were identified by surveillance doppler ultrasonography. The preangioplasty and postangioplasty pressure gradients were recorded. The therapeutic results were monitored by the follow up of the clinical symptoms, the laboratory values, CT and ultrasonography.
Results
The overall technical success rate was 100%. The clinical success rate was 83% (5/6). A total of eight sessions of balloon angioplasty were performed in six patients. The mean pressure gradient decreased from 14.5 mmHg to 2.8 mmHg before and after treatment, respectively. The follow up periods ranged from three months to 64 months (mean period; 32 months). Portal venous patency was maintained in all six patients until the final follow up. Combined hepatic venous stenosis was seen in one patient who was treated with stent placement. One patient showed puncture tract bleeding, and this patient was treated with coil embolization of the right portal puncture tract via the left transhepatic portal venous approach.
Anastomotic stenosis of the portal vein is a relatively infrequent vascular complication that follows liver transplantation, and it is a potentially serious complication that can possibly lead to graft loss (1, 2). With the increasing incidence of living related or reduced size liver transplantation, percutaneous transhepatic angioplasty and stent insertion of the portal vein have gained world-wide acceptance as a viable therapeutic alternative for these patients (3, 4).
There have been a few reports that have dealt with the intermediate term follow-up results of angioplasty for portal vein stenosis after liver transplantation (5, 6). However, there have been very few reports on the mid-term and long-term follow up for post-transplant portal venoplasty, and especially for the results of this procedure in the Asian population.
The purpose of this study was to evaluate the mid-term follow up on the therapeutic responses to balloon angioplasty for portal venous stenosis after liver transplantation.
Of the 420 consecutive liver transplantations performed from May, 1996 to February, 2005, six patients (five males and one female) underwent percutaneous transhepatic angioplasty of the portal vein due to anastomotic stenosis. Of the six patients, two of them were children and the others were adults. The patients ranged in age from one year to 53 years (mean age; 27 years). Five patients received transplants from living related donors and one patient underwent orthotopic liver transplantation. For the patients who underwent living related liver transplantation, three patients received the left liver and two patients received the right liver. The diseases underlying these liver transplantations were as follows; three cases of liver cirrhosis, one case of hepatocellular carcinoma and two cases of biliary atresia.
The patients suffering with portal venous stenosis after liver transplantation were identified by their clinical signs and symptoms of portal hypertension or by surveillance Doppler ultrasonography. The three patients who were without any specific clinical manifestations were identified on routine Doppler ultrasonography. Three patients showed clinical symptoms and ascites. All the clinically symptomatic patients also underwent Doppler sonography and this confirmed their portal stenosis. The summary of the patients is shown in Table 1.
The criteria of Doppler sonography for the detection of portal vein stenosis were more than a 50% narrowing of the stenotic segment diameter compared with the main portal vein on the gray scale images in the adults, and a portal vein segment diameter below 2.5 mm in the pediatric patients. An increase of the portal flow velocity to more than 40 cm/sec at the anastomosis site or a postanastomotic jet flow noted on the Doppler US was also used as the diagnostic criteria for portal vein stenosis.
Technical success of the procedure was defined as less than 30% residual stenosis being observed on venography with the absence of variceal filling or collaterals. All the stenoses were located at the extrahepatic portal vein anastomosis sites. The ratio of the stenotic segment to the patent main portal vein was measured for each patient.
An informed consent was obtained from all the patients or their guardians. The procedures were performed under local anesthesia for four patients. The two pediatric patients had their procedures performed under general anesthesia. The transplanted liver was punctured with a 22G Chiba needle under fluoroscopic and ultrasound guidance, and the needle was targeted to the peripheral branch of the subcapsular portal vein. After achieving confirmation of the portal puncture with a test dose injection of contrast media, a 0.018-inch platinum-coated nitinol guide wire (M.I. Tech, Seoul, Korea) was advanced into the main portal vein. After changing the nitinol guide wire to a 0.035-inch guide wire (Glidewire; Terumo, Tokyo, Japan), a 6-8 Fr vascular sheath (COOK, Bloomington, IN, USA) was inserted. The portal venogram was obtained with a 5Fr Kumpe catheter (COOK, Bloomington, IN, USA). The portal pressure was measured at the postanastomotic main portal vein or at the level of the hepatic hilar portal bifurcation. A disposable pressure monitoring kit (Biosensors international, Singapore) was used for measuring the portal pressure.
The stenotic segment was passed with the guide wire and the prestenotic portal pressure was also measured. Angioplasty was performed with the same diameter or with a 10% larger diameter balloon than the diameter of the nonstenotic extrahepatic portal vein. Careful serial elevation of the balloon pressure was achieved by using inflation devices (Merit Medical Systems, South Jordan, UT, USA), and the balloon dilatation was continued until there was a loss of the balloon's waist. The elevated balloon pressure at the fully dilated state was maintained for 2 minutes. No intravenous or systemic heparinization was used. Postangioplasty anticoagulation was achieved by oral administration of aspirin 100 mg with or without dipyridamole 25 mg per day. After the balloon dilatation was completed, the postangioplasty portogram was obtained and the puncture tract was embolized with gelfoam (Cutanplast, Mascia Brunelli, Milano, Italy) pledgets through the sidearm of the vascular sheath or the angiographic catheter.
The clinical signs and symptoms, the laboratory values including the liver function test, and the radiologic monitoring of the Doppler sonographic results after the balloon dilatation of the portal vein were performed.
The technical success rate of the portal vein angioplasty was 100% (6/6). The clinical success rate was 83% (5/6). A total of eight sessions of portal vein angioplasty were performed in six patients. In two patients, two sessions of angioplasty were performed and the recurrent stenoses developed at 21 months and 31 months, respectively, from the time of the initial angioplasty. In one pediatric patient who had recurrent portal venous stenosis, the balloon diameter was increased up to 30% more than the initial procedure, and this stenosis was due to the age-related growth of the portal vein that was noted at the time of the second procedure. The repeated angioplasty procedures led to clinical and angiographic improvement in these two patients.
The balloon diameters ranged from 4-mm to 24-mm. The diameters of the stenotic segments ranged from 1.4 mm-5 mm (mean; 2.9 mm) and the percent of stenosis ranged from 75% to 85% (mean; 79%). The portal vein stenoses occurred at between one and 31 months after liver transplantation (mean period; 13.3 months) (Fig. 1).
The ratios of the stenotic segment to the main portal vein were elevated to be from 0.2 to 0.66 before and after treatment, respectively. The mean pressure gradient of the portal vein before treatment was 14.5 mmHg and this was decreased to 2.8 mmHg immediate after balloon dilatation. In four patients, the difference of the pressure gradients before and after angioplasty was 10-23 mmHg (mean difference; 16.2 mmHg). In the other two patients, the differences were 4 mmHg and 1 mmHg, respectively. All the patients showed poststenotic dilatation of the intrahepatic portal vein on portal venography.
Portal vein thrombosis was seen in one patient and we attempted to macerate the thrombus with the Hydrolyzer thrombectomy catheter (Cordis, J&J Medical Systems, FL, USA) system. However, this procedure was ineffective in removing the thrombus, so we successfully macerated the thrombus by using a large bore balloon (18 mm/4 cm) catheter.
A complication occurred for one pediatric patient. This patient received the left lobe lateral segment from the donor and the transplanted left liver wholly occupied the original liver bed from the right upper abdomen to the midabdomen. The patient showed massive postprocedural bleeding through the portal puncture tract at the right flank in spite of performing gelfoam embolization of the tract. Coil embolization of the portal puncture tract and the adjacent peripheral portal branch was performed by using the left transhepatic portal approach, and the bleeding was finally stopped.
One patient showed a subsequent increase in the amount of ascites in spite of the successful portal venoplasty. The CT scan revealed a combined hepatic venous stenosis and it was treated with the insertion of a 14 mm diameter/4 cm length self-expandable metallic stent (SMART Control, Cordis, J&J Medical Systems, Miami Lakes, FL, USA). The ascites decreased thereafter and the elevated liver enzymes were normalized. However, this patient showed further deterioration of the liver function in spite of the hepatic venous stent placement. Repeated portal venograms were performed, but portal vein flow and lumen were observed to be well preserved. This deterioration finally led to graft failure.
The length of the follow-up period ranged from three months to 64 months (mean follow up; 32 months). Five patients were completely free of their clinical symptoms after their portal vein angioplasty. Postprocedural follow up with doppler ultrasonography was performed in all six patients and CT follow up was performed in three patients. In four of the six patients, preprocedural doppler sonography showed a mean peak flow velocity of 125 cm/sec in the stenotic segment of the portal vein. On the follow up doppler sonography after portal venoplasty, we observed the preservation of the dilated portal vein diameter and a stable continuous wave on the spectral Doppler with less than 40 cm/sec of peak flow velocity at the dilated segment in all four patients. The other two patients showed widely patent portal veins on their follow up CT scans (Fig. 2).
Portal vein complications after liver transplantation, including anastomotic stenosis or thrombosis, are relatively uncommon vascular complications that occur in only 0.5-3% of patients (7-9). The incidence of these complications is high for pediatric patients, for reduced-size liver transplantation and for patients requiring intraoperative reconstruction of the portal vein due to the discrepancy of the vessel's sizes, the limited length of the donor portal vein and the grafts that are prone to delayed stenosis (6, 8). Although these complications have been traditionally treated by such surgical interventions as retransplantation, portacaval shunting or resection and reconstruction of the anastomosis, such interventions are technically difficult for surgeon to perform due to the postsurgical fibrosis around the graft and the several complicating factors that accompany this procedure (9-11).
Since the first report of portal venoplasty by Raby et al. (12), percutaneous balloon angioplasty for the portal vein stenosis that occurs after liver transplantation has been a widely accepted procedure for alleviating the symptoms of portal hypertension and for preserving the graft.
Although the long term follow up results of portal venoplasty after liver transplatation in children and adolescents have already been reported on by Funaki et al. (13), we now have some limited data regarding the mid-term and long-term results. The mid-term results of portal vein angioplasty after liver transplantation, as reported by several authors, have dealt with the 28-33 month follow up data (5, 6). Our study included both full-grown adults and pediatric patients, and we presented the relatively long-term follow up until 64 months. The patency of the portal vein was well maintained in all the patients without any clinical deterioration being noted.
Thirty-three percentage of the patients in this study required repeated sessions of portal venoplasty after liver transplantation without the placement of stents. In one patient who had recurrent stenosis of the portal vein, there was no hemodynamically significant decrease of the transstenotic pressure gradients. There was only a 4 mmHg decrease in the pressure gradient after portal venoplasty in this patient. Although some reports have considered a transstenotic pressure gradient of more than 5 mmHg as abnormal (2, 6, 8, 11), no standard guidelines for the significant pressure gradient have yet been established. In this patient with a 4 mmHg gradient, the post venoplasty gradient was 0 mmHg and the clinical symptoms were improved, along with the good follow up Doppler sonography results. However, the other patient with a 1 mmHg post-venoplasty gradient showed further deterioration of the liver function, and this led to graft failure. Godoy et al. have reported on a case where in spite of the hemodynamically significant pressure drop (14 mmHg to 1 mmHg) after portal venoplasty, the clinical symptoms persisted (2). In addition, some authors believe that anastomotic stenosis of the portal vein will respond differently to percutaneous balloon angioplasty depending on their cause, the size of the stenosis and the patients' ages (6). Therefore, the transstenotic pressure gradient does not seem to have any direct correlation with the clinical and therapeutic results. As seen in our case, portal venoplasty might not be so helpful for patients whose clinical symptoms are possibly related with graft dysfunction and not with the stenosis. One pediatric patient showed restenosis of the portal vein nine months from the first intervention, and the 24 months follow-up CT after the second angioplasty showed a well-preserved portal vein lumen and there was good function of the graft.
One patient in our study showed a combined portal venous thrombosis with their stenosis. The reports in the literatures have shown that there is a high incidence of portal vein thrombosis when the vascular conduits are used to reconstruct the donor's and the recipient's portal veins (13, 14). We attempted using the Hydrolyzer Thrombectomy Catheter system to macerate the thrombus, but the procedure was not successful. Portal vein occlusion or thrombosis after liver transplantation has been treated by a combination of thrombolysis, stent placement and mechanical fragmentation (15-17). Therefore, we performed a large profile balloon (18 mm/4 cm) dilatation in the main portal vein to achieve both thrombus maceration and the portal vein dilatation. The follow up Doppler ultrasonography showed that the small fragmented thrombi had dislodged themselves into the intrahepatic portal veins, and these were clinically insignifcant. The fragmented thrombi completely disappeared on the final follow up sonography.
Although the number of patients in our study was small, our series showed clinical and therapeutic results equivalent to or slightly better that the previously published midterm and long-term data regarding portal venoplasty after liver transplantation. Metallic stenting was not definitely indicated for the cases with recurred stenosis in our series. The two patients who underwent a second session of angioplasty maintained portal patency during the 26 months and the 33 months follow up from the time of the second session portal venoplasty, respectively. One patient showed an unrelated graft dysfunction two months after our initial intervention. However, the clinical responses to balloon dilatation of portal venous stenosis after liver transplantation can be unpredictable. Morphologically and hemodynamically failed angioplasty or the immediate recoiling of the stenotic segment will require placement of a metallic stent. Because physicians' total experience with posttransplantational portal stenosis is somewhat limited, larger clinical studies are needed that will include long term follow up.
In conclusion, portal venous angioplasty after liver transplantation is a safe procedure for alleviating the signs and symptoms of portal hypertension. Although stent placement is a limited option for failed angioplasty, repeated balloon dilatation is helpful for the majority of cases.
Figures and Tables
Fig. 1
A 33-year-old male patient showed main portal vein stenosis after 31 months from liver transplantation.
A. The large thrombus (arrow) is seen in the prestenotic main portal vein.
B. Portal venoplasty was performed via the full dilatation of an 18 mm balloon. The transstenotic pressure gradient was 4 mmHg.
C. Postangioplasty venogram shows residual stenosis of up to 50%. Residual thrombus in the main portal vein (blank arrow) appears as a filling defect. Collateral venous engorgement is still seen (curved arrow).
D. Dual balloon angioplasty was performed up to a 24 mm diameter (with two 12 mm balloons).
E. Final portal venogram showed marked decrease of the collateral vein backflow and the widened main portal vein lumen. Thrombus is not seen in the main portal vein. The transstenotic pressure gradient decreased to 0 mmHg.
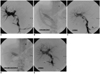
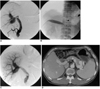
Fig. 2
A 33-year-old male patient developed portal vein stenosis 2 months after living related liver transplantation.
A. The portal vein shows a severe focal tight stenosis (curved arrow). Note the venous backflow (blank arrow).
B. A 16 mm balloon angioplasty was performed.
C. The immediate portal venogram shows good main portal vein flow without significant residual stenosis. The venous backflow has disappeared. The transstenotic pressure gradient was decreased from 26 mmHg to 3 mmHg.
D. The 8 month follow up CT shows a widely patent lumen of the main portal vein.
References
1. Wozney P, Zajko AB, Bron KM, Point S, Starzl TE. Vascular complications after liver transplantation: a 5-year experience. AJR Am J Roentgenol. 1986. 147:657–663.
2. Godoy MA, Camunez F, Echenagusia A, Simo G, Urbano J, Calleja J, et al. Percutaneous treatment of benign portal vein stenosis after liver transplantation. J Vasc Interv Radiol. 1996. 7:273–276.
3. Buell JF, Funaki B, Cronin DC, Yoshida A, Perlman MK, Lorenz J, et al. Long-term venous complications after full-size and segmental pediatric liver transplantation. Ann Surg. 2002. 236:658–666.
4. Vignali C, Cioni R, Petruzzi P, Cicorelli A, Bargellini I, Perri M, et al. Role of interventional radiology in the management of vascular complications after liver transplantation. Transplant Proc. 2004. 36:552–554.
5. Funaki B, Rosenblum JD, Leef JA, Hackworth CA, Szymski GX, Alonso EM. Angioplasty treatment of portal vein stenosis in children with segmental liver transplants: mid-term results. AJR Am J Roentgenol. 1997. 169:551–554.
6. Zajko AB, Sheng R, Bron K, Reyes J, Nour B, Tzakis A. Percutaneous transluminal angioplasty of venous anastomotic stenoses complicating liver transplantation: intermediate-term results. J Vasc Interv Radiol. 1994. 5:121–126.
7. Lerut J, Tzakis AG, Bron K, Gordon RD, Iwatsuki S, Esquivel CO, et al. Complications of venous reconstruction in human orthotopic liver trnasplantation. Ann Surg. 1987. 205:404–414.
8. Rollins NK, Sheffield EG, Andrews WS. Portal vein stenosis complicating liver transplantation in children: percutaneous transhepatic angioplasty. Radiology. 1992. 182:731–734.
9. Scantlebury VP, Zajko AB, Esquivel CO, Marino IR, Starzl TE. Successful reconstruction of late portal stenosis after hepatic transplantation. Arch Surg. 1989. 124:503–505.
10. Rouch DA, Emond JC, Ferrari M, Yousefzadeh D, Whitington P, Broelsch CE. The successful management of portal vein thrombosis after hepatic transplantation with splenorenal shunt. Surg Gynecol Obstet. 1988. 166:311–316.
11. Funaki B, Rosenblum JD, Leef JA, Hackworth CA, Szymski GX, Alonso EM, et al. Portal vein stenosis in children with segmental liver transplants: treatment with percutaneous transhepatic venoplasty. AJR Am J Roentgenol. 1995. 165:161–165.
12. Raby N, Karani J, Thomas S, O'Grady J, Williams R. Stenoses of vascular anastomoses after hepatic transplantation: treatment with balloon angioplasty. AJR Am J Roentgenol. 1991. 157:167–171.
13. Funaki B, Rosenblum JD, Leef JA, Zaleski GX, Farrell T, Lorenz J, et al. Percutaneous treatment of portal venous stenosis in children and adolescents with segmental hepatic transplants: long-term results. Radiology. 2000. 215:147–151.
14. Millis JM, Seaman DS, Piper JB, Alonso EM, Kelly S, Hackworth CA, et al. Portal vein thrombosis and stenosis in pediatric liver transplantation. Transplantation. 1996. 62:748.
15. Denys A, Chevallier P, Doenz F, Qanadli SD, Sommacale D, Gillet M, et al. Interventional radiology in the management of complications after liver transplantation. Eur Radiol. 2004. 14:431–439.
16. Bhattacharjya T, Olliff SP, Bhattacharjya S, Mirza DF, McMaster P. Percutaneous portal vein thrombolysis and endovascular stent for management of post transplant portal venous conduit thrombosis. Transplantation. 2000. 69:2195–2198.
17. Baccarani U, Gasparini D, Risaliti A, Vianello V, Adani GL, Sainz M, et al. Percutaneous mechanical fragmentation and stent placement for the treatment of early post transplantation portal vein thrombosis. Transplantation. 2001. 72:1572–1582.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download