This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
The purpose of our study was to assess whether a review of multiphasic helical CT combined with the previous serial CT images could be helpful to depict a viable tumor in hepatocellular carcinoma treated with transarterial chemoembolization.
Materials and Methods
Twenty-four consecutive patients with 35 hepatocellular carcinomas underwent transarterial chemoembolization followed by hepatic resection. First, three radiologists independently analyzed the last CT images taken before resection for the presence of viable tumor. A second analysis was then performed using the last CT combined with the previous serial CT images. The CT analyses were then compared with the pathologic results. The added value of the review of the previous serial CT images was evaluated by performing a receiver operating characteristic analysis. The sensitivity, specificity and diagnostic accuracy for the depiction of viable tumor were also assessed, and the characteristics of the false-negative lesions were pathologically evaluated.
Results
The mean diagnostic accuracies (Az values) for the depiction of viable tumor with using the last CT alone and with the review of the previous serial CT images for all observers were 0.885 and 0.901, respectively, which were not significantly difference (p > 0.05). However, the additional review of the previous serial CT images allowed the observers to render a correct diagnosis for three lesions that had been incorrectly diagnosed with the review of last CT alone. The sensitivity, specificity and diagnostic accuracy of the last CT along with the review of the previous serial CT images were 78%, 97% and 84%, respectively. All of the 16 false-negative lesions diagnosed by each observer showed 90% or greater necrosis on the pathologic examination.
Conclusion
For the depiction of viable tumor in hepatocellular carcinoma treated with transarterial chemoembolization, although the difference in the diagnostic accuracies was not statistically significant, a review of the multiphasic helical CT combined with the previous serial CT images could help reach a correct diagnosis for those lesions incorrectly diagnosed with the review of the last CT alone.
Keywords: Liver, CT, Liver neoplasm, CT
The utility of multiphasic (biphasic or triphasic) helical CT as a first-line diagnostic modality for the detection of hepatocellular carcinoma has been evaluated (
1-
3). This modality has also been used to evaluate the therapeutic efficacy and the possibility of residual viable tumor or recurrent tumor after patients were treated with transarterial chemoembolization for hepatocellular carcinoma (
4).
It is sometimes difficult to detect any remaining viable tumor and tumor recurrence because various lesions and phenomena in the liver show suspicious high attenuation within or around the iodized oil-containing tumor on the hepatic arterial phase, and this can mimic tumor enhancement (
3,
4). According to a recent study (
4), the addition of unenhanced CT images could increase the diagnostic confidence for the assessment of viable tumor, compared with using just the biphasic enhanced CT images, in patients treated with transarterial chemoembolization for hepatocellular carcinoma.
In the cases where the findings on the last CT, including the unenhanced images, are inconclusive, we questioned the value of reviewing the previous serial CT images. Therefore, we retrospectively evaluated whether a review of the multiphasic helical CT combined with previous serial CT images could be helpful for depicting a viable tumor in hepatocellular carcinoma treated with transarterial chemoembolization.
MATERIALS AND METHODS
Patient Selection
From October 1996 to November 2002, 30 consecutive patients with hepatocellular carcinomas underwent transarterial chemoembolization followed by hepatic resection at our institution. Of these patients, we included the patients who met the two following criteria: (a) two or more four-phase (i.e., hepatic arterial, portal venous, and delayed phase, and unenhanced image) helical CTs were taken after the first transarterial chemoembolization, and (b) hepatic resection was done within one month of the last follow-up CT. Finally, 24 patents with 35 hepatocellular carcinomas were included in this study. Eighteen patients had one tumor, four patients had two tumors, one patient had three tumors and one patient had six tumors. They underwent lobectomy (n = 22), segmentectomy (n = 1) or liver transplantation (n = 1) after transarterial chemoembolization. Of these, 20 patients could undergo hepatic resection because they had recovered enough hepatic function to endure hepatic surgery, as compared to the time when they had undergone the initial transarterial chemoembolization. There were 21 men and three women, and their ages ranged from 38 to 73 years (mean age; 54 years). This study was approved by the institutional review board of our hospital.
All the patents in the study had liver cirrhosis as a result of either hepatitis B (n = 23), or hepatitis C (n = 1). Twenty-six hepatocellular carcinomas were diagnosed by the pathologic results for those patients that demonstrated viable tumor in the resected specimens. Of the nine totally necrotic tumors, the diagnosis of three hepatocellular carcinomas was established based on the results of percutaneous needle biopsy. The remaining six hepatocellular carcinomas showed the characteristic laboratory findings (e.g., elevated α-fetoprotein levels and viral markers) in combination with the characteristic radiological findings on the follow-up CT images. The mean diameter of the hepatocellular carcinomas was 3.7 cm (range; 0.5-12 cm).
Seventeen patients underwent multiple treatments of transarterial chemoembolization (range; 2-5 treatments, mean; 2.9 treatments). The remaining seven patients underwent it once. The mean interval between the transarterial chemoembolization and the follow-up CT was 20 days (range; 3-23 days), and the mean interval between the last CT and surgery was 14 days (range; 1-30 days).
Multiphasic Helical CT
The CT scans were performed with a helical scanner (HiSpeed Advantage; GE Medical Systems, Milwaukee, WI, USA). The scanning parameters were 120 kVp, 180 mAs, 7 mm section collimation and a 7 mm/sec table speed during a single breath-hold helical acquisition of 25-30 seconds, depending upon the liver size. The images were obtained in a craniocaudal direction and they were reconstructed every 7 mm to provide contiguous or overlapping sections for the unenhanced and enhanced images. After the acquisition of the unenhanced images, the hepatic arterial phase, the portal venous phase and the delayed phase images were obtained with delays of 30, 60 and 180 seconds, respectively, after the start of injecting 120 mL of nonionic iodinated contrast material (Iopamiro 300, Bracco, Milano, Italy and Ultravist 300, Schering, Berlin, Germany) through the antecubital vein at a rate of 3 mL/sec.
Transarterial Chemoembolization Techniques
Transarterial chemoembolization was performed with a mixture of iodized oil (Lipiodol; Guerbet, Aulnay-sous-Bois, France) and doxorubicin hydrochloride (Adriamycin; Kyowa Hakko Kogyo, Tokyo, Japan) in all the patients. We used 3 mg of doxorubicin hydrochloride and 1 cc of iodized oil per 1-cm diameter of the tumor. For particle embolization, we also used Gelfoam powder (Upjohn, Kalamazoo, MI, USA) if a patient had neither Child class C cirrhosis nor thrombosis in the major portal vein. The injection was continued until stasis was identified in the feeding artery. Infusion and embolization was performed with the catheter in a lobar or segmental artery.
Pathologic Analysis
All surgical specimens were reviewed by an experienced liver pathologist. The pathologic specimens were sectioned at 5-mm intervals and the tumor necrosis was estimated as a percentage. The percentage of tumor necrosis was determined after investigating all the sections. The pathologic findings that were used as the standards of reference revealed that nine of the 35 hepatocellular carcinomas were totally necrotic.
Image Analysis
The CT images were retrospectively and independently reviewed by three radiologists. One observer had 24 years experience at abdominal imaging and other two observers had five years experience each. The observers were informed of the patients' histories in as much as they knew that all the patients had undergone transarterial chemoembolization for hepatocellular carcinoma, but the observers were blinded to the pathologic findings concerning the presence of viable tumor in each patient. First, the observers reviewed the last four-phase CT taken before liver resection. Second, the observers repeated the review two weeks after the first interpretation with using the last CT combined with the previous serial CT images. The observers were obliged to review the second-to-the-last CT. Further review of the other previous CT examinations was determined by the respective decision of the observers for each case.
All images were evaluated using a 2,000 × 2,000 Picture Archiving and Communication Systems (PACS; GE Medical Systems Integrated Imaging Solutions, Mt Prospect, IL, USA) monitor. The images were initially evaluated using two window settings (window level; 60 HU, window width; 350 HU, and window level; 110 HU, window width; 200 HU), and then the window settings were adjusted as needed. Images were interpreted for the presence, number, size and site of the viable tumors.
The image review was conducted on a tumor-by-tumor basis. Each observer also recorded his or her degree of confidence as to whether a lesion seen on an image represented a viable tumor. The attenuation of each lesion in relation to that of the liver (i.e., hypoattenuation, hyperattenuation, isoattenuation or mixed attenuation) was subjectively assessed. The diagnostic confidence for each lesion was scored using a five-point scale (1, not viable tumor; 2, probably not viable tumor; 3, possibly viable tumor; 4, probably viable tumor; 5, definitely viable tumor) at each interpretation session.
For the objectivity and reproducibility of the image analysis performed in this study, the important criteria for a viable tumor and for the noncancerous lesions were determined. We regarded the following lesions as viable areas of tumors on the last CT images: (a) a hyperattenuating or isoattenuating lesion seen during the hepatic arterial phase and as a hypoattenuating lesion seen during the portal venous phase or the delayed phase, (b) an area of mixed attenuation seen on the hepatic arterial phase images that showed a hypoattenuating portion either on the portal venous phase or the delayed phase, and (c) a nodule that was seen as being isoattenuation on the hepatic arterial phase, the portal venous phase or the delayed phase images, but it was seen as hypoattenuation on the unenhanced images (
4). A lesion that showed wedge-shaped hyperattenuation during the hepatic arterial phase and that appeared as isoattenuation on the portal venous phase, on the delayed phase images and on the unenhanced images was considered to be a noncancerous lesion.
When the last CT along with the previous serial CT images was reviewed, a lesion that showed a newly presenting defect within an iodized oil-containing nodule was regarded as a viable portion of tumor. A small peritumoral area with suspicious enhancement on the hepatic arterial phase of the last CT, which showed an iodized oil-containing liver parenchyma on the previous serial CT images, was considered as an arterioportal shunt and not as a viable tumor. Also, a long-standing hypoattenuating area (longer than one year) that did not change after contrast enhancement within a nodule of hepatocellular carcinoma was not considered viable tumor.
A radiologist (the study coordinator) and a pathologist reviewed the pathologic findings and their correlation with the multiphasic CT findings. They evaluated the location (upper or lower, right or left, and anterior or posterior) and the size of the viable portions within the tumors on both the CT and the pathologic examinations.
Statistical Analysis
A binominal receiver operating characteristic (ROC) curve was calculated for each observer's confidence rating data with using maximum-likelihood estimation. We evaluated the diagnostic accuracy of the last CT alone and the last CT combined with the previous serial CT images by calculating the area under each observer-specific binomial ROC curve (denoted as the Az index) (
5). The lesions that were assigned a score of three to five were considered as diagnosed viable tumors. The sensitivity and specificity were calculated for each observer and for each different review of the CTs, and the statistical analysis of their differences was assessed using the McNemar test (SPSS, version 10.0; SPSS, Chicago, IL, USA). The false negative rates for the detection of viable tumors were also calculated for the review of the last CT alone and for the review of the last CT along with the previous serial CT images. A
p value of less than 0.05 was considered to be statistically significant. κ statistics were used to assess the interobserver agreement for the presence of a viable portion of tumor with the review of the last CT alone and with the review of the last CT combined with the previous serial CT images. The degree of agreement was categorized as follows: κ values of 0.00-0.20 were considered to indicate poor agreement, κ values of 0.21-0.40 were considered to indicate fair agreement, κ values of 0.41-0.60 were considered to indicate moderate agreement, κ values of 0.61-0.80 were considered to indicate good agreement and κ values of 0.81-1.00 were considered to indicate excellent agreement (
5).
RESULTS
During the two analyses of the CT images, each observer detected all 35 hepatocellular carcinomas that were pathologically demonstrated in the resection specimens.
The mean diagnostic accuracies (Az values) for the depiction of viable tumor with the last CT alone and with the review of previous serial CT images for all the observers were 0.885 and 0.901, respectively (
p > 0.05) (
Table 1). The sensitivity, specificity and diagnostic accuracy of the last CT alone were 72%, 91% and 78%, respectively, and the corresponding values for the review of the last CT combined with the previous serial CT images were 78%, 97% and 84%, respectively (
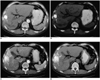
Table 2), and the differences were not statistically significant. Although these differences in the diagnostic accuracies were not statistically significant, the review of the previous images enabled the observers to render a correct diagnosis for three (9%) tumors. Two of these three tumors showed as being slightly hyperattenuating focal areas adjacent to the iodized oil-containing tumors on the hepatic arterial phase images of the last CT, and these two tumors appeared as isoattenuating areas on the unenhanced images. The second to the last CT showed that these areas appeared as parenchymal uptake around the iodized oil-containing tumors (
Fig. 1), and the two observers changed their confidence levels to probably not viable tumor. The tumors were totally necrotic on the pathologic examination. The one remaining tumor showed as an interval developed defect within the iodized oil-containing nodule that was not significantly enhanced on the last CT. On the basis of this feature, the two observers changed their confidence level to probably viable tumor. The pathology revealed a 70% necrotic hepatocellular carcinoma.
After reviewing of the last CT and the previous serial CT images, all three observers assessed the 10 compact iodized oil-containing tumors as being non-viable. Of these, eight were totally necrotic tumors that were confirmed on the pathologic findings. The two remaining tumors were 95% and 90% necrotic tumor, respectively. Thus, the compact iodized oil-containing tumors showed a mean necrosis rate of 98.5%.
All of the 16 false-negative lesions that were diagnosed by each observer after reviewing the last CT combined with the previous serial CT images showed 90% or greater necrosis on the pathologic examination (
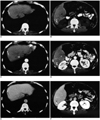
Table 3). Particularly, four tumors of these lesions were diagnosed as negative lesions simultaneously by all the observers with both the review of the last CT alone and the review of the last CT with the previous serial CT images. All of these four tumors were 1.5 cm in diameter or smaller. Two of these were compact iodized oil-containing tumors (
Fig. 2). The remaining two showed no definite enhancement in the iodized oil defect portion on the hepatic arterial phase CT.
The interobserver agreement (κ statistic) for the presence of a viable tumor was 0.828 to 0.943 for the review of the last CT alone and 0.826 to 0.885 for the additional review of the previous serial CT images (
Table 4). The interobserver agreement for the review of the last CT with or without the previous serial CT images was considered excellent.
DISCUSSION
The criteria suggested by the World Health Organization and that is used for evaluating the effect of chemotherapy on cancer cannot be applied to the evaluation of transarterial chemoembolization therapy because any tumor reduction can seldom be recognized before one month after the treatment (
6). Thus, other criteria have been evaluated to determine the efficacy of transarterial chemoembolization treatment for hepatocellular carcinoma by using iodized oil retention in a tumor and the enhancement on CT (
7,
8). After the initial remission following transarterial chemoembolization, Lee et al. (
9) suggested that the overall cumulative recurrence rate of hepatocellular carcinoma was 23% after one year, 55% after two years and 67% after three years, and 45% of the recurrences occurred adjacent to a primary site that was considered controlled by the transarterial chemoembolization. Hence, periodic CT follow-up or angiography might be recommended even though there may be the appearance of complete remission for hepatocellular carcinoma.
With the advent of helical CT and with the other rapid technical advances, multiphasic helical CT has recently become a more useful imaging modality for the detection and characterization of liver lesions, for the follow-up after local treatment or surgical excision, and for the assessment of the hemodynamic changes in the liver. However, to the best of our knowledge, there has been no previous study that has compared the preoperative CT and the review of the previous serial CT images with the resected specimens for the evaluation of the viable portion of hepatocellular carcinoma treated with transarterial chemoembolization.
In the past, some investigators have reported on the diagnostic efficacy of the hepatic arterial phase CT and the portal venous phase CT for the detection of hypervascular tumors, and especially hepatocellular carcinoma (
10,
11). Lim et al. (
1) have suggested that the delayed phase is important for the detection of small hepatocellular carcinomas that are less than 2 cm, for confirming or increasing the confidence level for the detection of equivocal nodules on the arterial or portal venous phase images (because those nodules are usually more conspicuous on the delayed phase than on the portal venous phase imaging), and for the differentiation of arterioportal shunting from the true hepatocellular carcinoma. In our study, this concept was used to depict a viable tumor among the arterioportal shunt areas.
For the hepatocellular carcinoma treated with transarterial chemoembolization, Takayasu et al. (
8) have suggested that the iodized oil deposition in a tumor could be considered as necrosis. Choi et al. (
7) have also proposed that the complete retention of iodized oil in a tumor and the surrounding liver demonstrated the best therapeutic effects. We adopted this principal criterion for the iodized oil retention in our study.
Kim et al. (
4) have recently pointed out that unenhanced images could be of additional diagnostic value to supplement the enhanced biphasic helical CT images for the patients treated with transarterial chemoembolization for hepatocellular carcinoma by facilitating the differentiation of the true lesions from the hyperattenuating pseudolesions that simulate viable tumor. Further, they stated that these unenhanced images can also aid in the detection of any isoattenuation lesion on the hepatic arterial phase and the portal venous phase because of the hypoattenuation on the unenhanced images. We also evaluated the unenhanced images, and we used the criteria suggested by Kim et al. (
4).
In addition to the criteria in the previous studies (
1,
4,
7,
10,
11), we considered the long-standing hypoattenuating areas within an iodized nodule on the enhanced images of the serial CT images as necrotic lesion despites a lack of iodized oil. On the other hand, a lesion that showed an interval developed defect within an iodized nodule was considered as a viable portion of tumor.
In our study, although no significant statistical difference was found between the review of the last CT alone and the review of the last CT along with the previous serial CT images, the additional review of the previous serial CT images allowed the observers to render a correct diagnosis for three (9%) lesions. We believe that many abdominal radiologists actually do review some of the previous CT images when they find an equivocal viable lesion on the liver CT following transarterial chemoembolization.
According to our results, after the review of the last CT combined with the previous serial CT images, all the false-negative lesions showed 90% or greater necrosis on the pathologic examination. Thus, if a substantial (larger than 10%) viable tumor is present in an iodized nodule, we can depict it on CT with a thorough review of the previous serial CT images.
Our study has some limitations. First, as this is a retrospective study, all the patients of the study group did not undergo regular follow-up CT and transarterial chemoembolization because of suspected complications or because of personal preference. Second, we included a relatively small number of hepatocellular carcinomas, and so this might show no significant statistical difference between the review of the last CT alone and the review of the last CT along with the previous serial CT images. Finally, although we thoroughly reviewed the pathologic specimens alongside the CT images, any perfect area-by-area histopathologic correlation was actually impossible. By referring to the location and size of viable tumors within the tumors, however, we tried to match the CT and histopathologic findings as closely as possible. In our results, two of the three tumors that had a correct diagnosis rendered after the review of previous images appeared as parenchymal uptake around the iodized oil-containing tumors on the second to the last CT. The pathology demonstrated totally necrotic tumors, and this revealed that the suspicious hyperattenuating focal areas adjacent to the iodized oil-containing tumors on the hepatic arterial phase images of the last CT were pseudolesion. For the third hepatocellular carcinoma with 70% necrosis, we could not perfectly match the interval developed defect within the iodized oil-containing nodule and the 30% viable tumor portion on the pathologic specimen. Also, we do not know the reason why the two compact iodized oil-containing tumors seen on CT had viable tumor portions on the pathology examination.
In conclusion, for depicting the viable tumor in hepatocellular carcinoma patients treated with transarterial chemoembolization, although the difference in the diagnostic accuracies was not found to be statistically significant in our study, a review of the multiphasic helical CT combined with the previous serial CT images can help render a correct diagnosis for those lesions incorrectly diagnosed with the review of only the last CT.







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download