Abstract
Objective
To demonstrate the functional neuroanatomy associated with sexual arousal visually evoked in depressed males who have underlying sexual dysfunction using Blood Oxygenation Level Dependent-based fMRI.
Materials and Methods
Ten healthy volunteers (age range 21-55: mean 32.5 years), and 10 depressed subjects (age range 23-51: mean 34.4 years, mean Beck Depression Inventory score of 39.6±5.9, mean Hamilton Rating Scale Depression (HAMD)-17 score of 33.5±6.0) with sexual arousal dysfunction viewed erotic and neutral video films during functional magnetic resonance imaging (fMRI) with 1.5 T MR scanner (GE Signa Horizon). The fMRI data were obtained from 7 oblique planes using gradient-echo EPI (flip angle/TR/TE=90°/6000 ms/50 ms). The visual stimulation paradigm began with 60 sec of black screen, 150 sec of neutral stimulation with a documentary video film, 30 sec of black screen, 150 sec of sexual stimulation with an erotic video film followed by 30 sec of black screen. The brain activation maps and their quantification were analyzed by SPM99 program.
Results
There was a significant difference of brain activation between two groups during visual sexual stimulation. In depressed subjects, the level of activation during the visually evoked sexual arousal was significantly less than that of healthy volunteers, especially in the cerebrocortical areas of the hypothalamus, thalamus, caudate nucleus, and inferior and superior temporal gyri. On the other hand, the cerebral activation patterns during the neutral condition in both groups showed no significant differences (p < 0.01).
Conclusion
This study is the first demonstration of the functional neuroanatomy of the brain associated with sexual dysfunction in depressed patients using fMRI. In order to validate our physiological neuroscience results, further studies that would include patients with other disorders and sexual dysfunction, and depressed patients without sexual dysfunction and their treatment response are needed.
A number of investigators (1-6) have reported that depressed patients have difficulties in sexual function, including the loss of sexual interest, diminished ability to maintain sexual arousal or to achieve orgasm during an episode of major depression. However, there have been few clinical studies (4) reporting on the neuroanatomy associated with sexual arousal in depressed patients.
Recently, some few studies were enlisted healthy volunteers to identify the cerebral centers involved in sexual arousal by using functional imaging techniques that bridge the gap between neural systems and behavioral neurosciences. The representative tools for imaging functional neuroanatomy in vivo include: positron emission tomography (PET) (7, 8), single photon emission computed tomography (SPECT) (9), and functional magnetic resonance imaging (fMRI) (10-13). The nuclear medicine techniques such as PET and SPECT are the most sensitive imaging tools to evaluate biochemical processes in vivo, and these methods are relatively easy to quantify, particularly the PET studies, provided that an appropriate physiologic model is available. However, nuclear medicine imaging is not only an invasive device because of the use of radiopharmaceuticals, it is also an imaging method with relatively low spatial and temporal resolution of images compared to fMRI. Therefore, these nuclear medicine imaging techniques are potentially limited in clinical application for measuring physiological phenomenon having a fast time scale and precise neuroanotomical detail (14). On the other hand, fMRI is a noninvasive technique that is not limited in the number of scans available on a subject because there is no exposure to radioactive pharmaceunticals. Functional MRI is capable of detecting neuronal activity indirectly by measuring changes in signal intensity related to regional cerebral blood flow and local deoxyhemoglobin concentrations in an activated cortex (15). Also, the fMRI is likely to give more accurate neuroanatomical information on sexual arousal than does PET, and this is probably due to high spatial and temporal resolution of the fMRI.
The purpose of this study was to evaluate the main differences of functional neuroanatomy associated with sexual arousal when comparing between the healthy volunteer's response and depressed patients' underlying sexual dysfunction by using BOLD-based fMRI technique.
Ten depressed males with sexual arousal dysfunction (age range 23-51: mean 34.4 years, mean Beck Depression Inventory [BDI] score of 39.6±5.9, mean 17- item Hamilton Rating Scale Depression [HAMD]-17 score of 33.5±6.0), and ten healthy volunteers that were gender- and age-matched (age range 21-55: mean 32.5 years), participated in this study. All the participants signed informed consent and were asked to avoid sexual contact leading to orgasm for at least 24 hours before imaging, and they did not use any medications or mind affecting drugs. All healthy volunteers underwent a medical examination and psychiatric review, and they denied any past psychiatric pathology. Diagnostic evaluations for the MDD (Major Depressive Disorder) subjects included the Structured Clinical Interview for the DSM-IV (SCID), BDI, and HAMD-17.
The visual stimulation paradigm consisted of alternating periods of control and activations. It began with a 60 sec black screen followed by a 150 sec neutral stimulation with a documentary video film, 30 sec of black screen, a 150 sec sexual stimulation with a erotic video film, and 30 sec of black screen. The video films were presented to the subjects on a mirror located at the top of the head coil, and the video-images were received from the outside of the magnetic room.
Immediately after each fMRI acquisition, the participants were asked to answer the following question that they were to score from the range of 1 (no sexual arousal) to 10 (maximal sexual arousal): "To what degree were you sexually aroused?"
T1-weighted MR images (spin echo, flip angle/TR/TE=90°/500 ms/50 ms) were obtained for anatomical localization using a 1.5 T MRI scanner (GE medical systems, Milwaukee, Wis). The BOLD-contrast fMRI images were acquired from 7 slices covering the whole brain areas using gradient-echo EPI with 90° flip angle, 6000 ms TR, 50 ms TE, and 10 mm slice thickness. The functional MRI was generally well tolerated by all subjects, however, two depressive subjects and one healthy volunteer complained about the acoustic echo planar scanner noise.
Image reconstruction was performed by using the "MRIcrow1.36" (Univ. Nottingham) and SPM99 programs (The Wellcome Department of Cognitive Neurology, University College, London, UK) to realign and spatially normalize the images to a template brain that approximated the Talairach and Tournoux atlas spaces. The images were then smoothed with a Gaussian spatial filter with a full-width-at-half-maximum (FWHM) of 8 mm to increase the signal to noise ratio (SNR). The activation maps were generated by applying both the clustering threshold with a minimal clustering size of 27, based on three-dimensional search, and the t-threshold corresponding to a statistical level of p < 0.01.
Data analysis was focused on the comparison of brain activation patterns between depressed males and healthy male subjects; these brain activation patterns were evoked by a visual sexual arousal stimulus, and the analysis used independent two sample t-tests.
All of 10 volunteers were sexually aroused by visual stimulation, and they had a mean score of 7.8±1.6 on the 10-point scale, whereas the depressed subjects had a mean score of 3.7±1.1. None of the subjects from either group were sexually aroused by the documentary film.
Figure 1 demonstrates the differential activation between healthy (Fig. 1A) and depressed subjects (Fig. 1B) for their neural patterns. Figures 2 and 3 show the fMR images on the 19 contiguous axial slices associated with visually evoked sexual arousal in the healthy volunteers and depressed subjects, respectively. The brain activation patterns during the neutral conditions for both the healthy and depressed subjects showed no significant differences at the level of p < 0.01 (Fig. 1): the middle and inferior occipital gyrus, inferior temporal gyrus, inferior frontal gyrus, and thalamus were simultaneously activated in both cases.
In contrast, the level of activation in depressed subjects during the sexual arousal was significantly less than in the healthy volunteers, especially in the cortical regions of the hypothalamus, thalamus, caudate nucleus, and inferior and superior temporal gyri (refer to Table 1). It was interesting to note that the depressed subjects showed greater activation than did the normal volunteers in the middle and superior frontal areas (refer to Table 1).
In the past several years, a few functional imaging techniques focusing on the central nervous system have been utilized to study the neurophysiology of sexual arousal (8, 9, 11-14). Stoleru (8) and Redoute (16) have used PET to investigate changes in regional cerebral blood flow (rCBF) in male subjects presented with visual sexual stimuli. They reported that the right orbitofrontal cortex activation was correlated with both the cognitive and motivational components of their proposed model. They also suggested that the rostral portion of the anterior cingulate cortex (Brodmann area: BA 24) and the posterior portion of the hypothalamus activations were correlated with the autonomic component of sexual arousal, whereas activations in superior and middle frontal gyrus (BA 9) and anterior cingulate gyrus (BA 32) were related to the level of perceived emotion. Park (12, 13) and Karama (11) have recently used the BOLD fMRI technique to evaluate the cerebral center associated with the sexual arousal response in both males and females during viewing of erotic films. The significant activation patterns of the brain in male and female volunteers were similar to those of the PET studies (8, 14) mentioned before. However, there is no clinical report using fMRI in evaluating the functional neuroanatomy associated with sexual arousal for the depressed patients with sexual dysfunction. In this study, we have shown significant differences of brain activation between depressed patients and healthy volunteers during visually evoked sexual arousal. During sexual stimulation, the depressed patients experienced one half less sexual arousal than did healthy volunteers: the mean scores of sexual arousal on the 10-point scale were 7.8±1.6 and 3.7±1.1 in healthy volunteer and depressive subjects, respectively. The finding is consistent with the previous reports (1-6). Also, the level of activation was significantly less in depressed subjects than in healthy volunteers in the cortical areas: hypothalamus, thalamus, caudate nucleus, and inferior & superior temporal gyri. These areas are involved in the regulation of sexual behavior both in animals and humans. However, the depressed patients had greater activation in comparison with the healthy volunteer in the middle and superior frontal regions.
In animals, the hypothalamus has, perhaps, the most frequently claimed pivotal role in the regulation of sexual behavior and physiological arousal (17, 18). In this study, activation of the hypothalamus in response to visually presented erotic stimuli is in agreement with other well-known findings (17-20), and this is also consistent with the result of a recent PET study (14) that demonstrated a correlation between activation in the hypothalamus and objective measures of penile tumescence. More specifically, the greater hypothalamic activation found in healthy volunteers could be viewed as suggesting that healthy volunteers were physiologically more aroused than were depressed subjects in response to the erotic video film.
The thalamus represents a hub that is capable of communicating with many important cortical areas for the integration of somatic and visceral function. The extensive thalamocortical interconnectivity has been theorized to constitute a neuronal basis for conscious awareness (21). In light of such a view, the greater sexual arousal experienced by healthy volunteers might be related to the fact that depressed subjects had significantly less thalamic activation. If this hypothesis were accurate, then the thalamus would be implicated in the cognitive dimension of sexual arousal.
Activation of the caudate nucleus is correlated with the urge to perform handwashing rituals in patients suffering from obsessive-compulsive disorder (22). McGuire (22) noted that during viewing of an erotic film, subjects were simultaneously confronted with the urge to act and with the impossibility to do so, hence suggesting a role for the ventral striatum in the control of the motor expression of sexual arousal, that is, in withholding the motor output of sexual arousal. Such a role could be implemented through the anatomic projections that the striatum receives from the cognitive subdivision of the anterior cingulate cortex (23).
Activation of the occipitotemporal area is in accord with the results of recent functional neuroimaging studies showing that, when compared to neutral visual stimuli, emotionally laden visual stimuli elicit increased activation in this cortical region (24-26). Assuming that viewing emotionally laden stimuli automatically elicits increased attentional tapping, the occipitotemporal activation noted in this study would be consistent with the hypothesis that attention to visual stimuli can modulate neural activity in the extrastriate visual cortex (27-32). On the contrary, activation during the neutral condition showed a similar pattern between two groups. Especially, the temporal and occipital lobes were highly activated. The visual association cortices are involved in the perception of visual stimulation. Various areas of the cerebral cortex are functionally related to the thalamic nuclei. The occipital and temporal lobes have extensive connections with the pulvinar nucleus of the thalamus and are involved in the processing of visual and auditory information (33).
In the region of middle and superior frontal gyri, the level of activation during the sexual arousal condition was significantly greater for depressed subjects than for the healthy volunteers. It is interesting to note that these areas are well-known cerebral cortices associated with major depressive disorders. The medial prefrontal cortex has previously been shown to be activated during the recall of happy, sad or disgusting moments in one's life or when viewing stimuli known to elicit these three emotional states (25). These findings led Lane et al. (25) to suggest that this region participates in aspects of emotional processing that is independent of valence, type or method of induction. Although the exact function of this cortical area remains to be elucidated, there is some evidence that the medial prefrontal cortex is involved in the conscious experience of emotion (34). Here, we must concur with Redoute et al. (16), that the activation seen in the medial prefrontal cortical region may be related to the level of perceived emotion and not to the sexual quality of emotion.
In this study, we have identified by using FMRI, for the first time, the functional neuroanatomy of the brain associated with sexual dysfunction in depressed patients. From this neuroanatomical and neurobiological perspective, we can presume that sexual dysfunction may be closely related to the pathogenesis of depressive symptoms; in addition the sexual dysfunction may have an important role in the neurobiology of major depressive disorders. However, additional correlative studies that would include depressed patients without sexual dysfunction and aftertreatment response are needed to clarify our findings of the functional neuroanatomy on sexual dysfunction in depressed patients. Also, objective measures of sexual arousal such as index of penile tumescence and a larger sample of subjects are needed to gain more informative results as well.
Figures and Tables
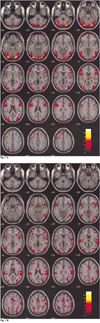 | Fig. 1Comparison of the brain activation patterns between a healthy volunteer (42 years old) (A) and a depressed subject (40 years old) (B) in neutral condition. The colored functional maps were overlaid on the T1-weighted MR images. Note that the level of activation is significantly stronger in a healthy volunteer than in a depressed patient, especially in the visual and cerebellar cortices. |
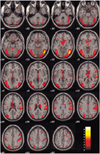 | Fig. 2Blood oxygenation level dependent-MR images on the 19 contiguous axial slices associated with visual sexual stimulation in a 42-year-old healthy volunteer. Color-coded pixels on the activation maps were scaled to the range between the cutoff-threshold (p < 0.05) and the highest t-score, 8.96. Prominent activation areas involve the occipital, temporal, cerebellar gyri, and the limbic systems associated with the regulation of sexual behaviors. |
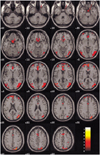 | Fig. 3Blood oxygenation level dependent-MR images on the 19 contiguous axial slices associated with visual sexual stimulation in a 40-year-old patient with depression. Color-coded pixels on the activation maps were scaled to the range between the cutoff-threshold (p < 0.05) and the highest t-score, 7.67. Activation is limited in the frontal and occipital cortices; any other limbic areas except part of the cingulate gyrus were not apparently activated in this case. |
Table 1
Comparison of t-scores for the Cerebrocortical Activation between Healthy Volunteers and Depressed Subjects during Visually Evoked Sexual Arousal

Note.-*The significance levels (p < 0.01) of the differences in activation (i.e., volume of the activated clusters) between the healthy volunteers and depressed subjects were determined by two sample t-test. **"Healthy > Depressed" indicates predominance of t-scores between healthy volunteers and depressed patients.
References
1. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960. 12:56–62.
2. Nelson JC, Charney DS. The symptoms of major depressive illness. Am J Psychiatry. 1981. 138:1–12.
3. Casper RC, Redmond DE, Katz MM, Schaffer CB, Davis JM, Koslow SH. Somatic symptoms in primary affective disorder. Arch Gen Psychiatry. 1985. 42:1098–1104.
4. Matthew RJ, Weinman ML. Sexual dysfunctions in depression. Arch Sex Behav. 1982. 11:323–328.
5. Tamburello A, Seppecher MF. Gemme R, Wheeler CC, editors. The effects of depression on sexual behavior: preliminary results of research. Progress in Sexology. 1977. New York, NY: Plenum Press;107–128.
6. Thase ME, Reynolds CF, Jennings JR, et al. Nocturnal penile tumescence is diminished in depressed men. Biol Psychiatry. 1988. 24:33–46.
7. George MS, Ketter TA, Post RM. SPECT and PET imaging in mood disorders. J Clin Psychiatry. 1993. 54:6–13.
8. Stoleru S, Gregoire M-C, Gerard D, et al. Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch Sex Behav. 1999. 28:1–21.
9. Ring HA, George M, Costa DC, Ell PJ. The use of cerebral activation procedures with single photon emission tomography. Eur J Nucl Med. 1991. 18:133–141.
10. Stehling MK, Turner R, Mansfield P. Echo-planar imaging; magnetic resonance imaging in a fraction of a second. Science. 1991. 254:43–50.
11. Karama S, Lecours AR, Leroux JM, et al. Areas of brain activation in males and females during viewing of erotic film excepts. Human Brain Mapping. 2002. 16:1–13.
12. Park K, Seo JJ, Kang HG, Ryu SB, Kim HJ, Jeong GW. A new potential of BOLD fMRI evaluating cerebral centers of penile erection. Int J Impot Res. 2001. 13:73–81.
13. Park K, Kang HG, Seo JJ, Kim HJ, Ryu SB, Jeong GW. Blood-Oxygenation-Level-Dependent fMRI for evaluating cerebral regions of female sexual arousal response. Urology. 2001. 57:1189–1194.
14. Nemeroff CB, Kilts CD, Berns GS. Functional brain imaging; twenty-first century phrenology or psychobiological advance for the millennium. Am J Psychiatry. 1999. 156:671–673.
15. Villringer A, Dirnagl U. Coupling of brain activity and cerebral blood flow; basis of functional neuroimaging. Cerebrovasc Brain Metab Rev. 1995. 7:240–276.
16. Redoute J, Stoleru S, Gregoire M-C, Costes N, Cinotti N, Lavenne F, et al. Brain processing of visual sexual stimuli in human males. Human Brain Mapping. 2000. 11:162–177.
17. Pfaff DW, Schwartz-Giblin S, McCarthy MM, Kow LM. Knobil E, Neill JD, editors. Cellular and molecular mechanisms of female reproductive behaviors. Physiology of reproduction. 1994. New York: Raven Press;107–220.
18. Sachs BD, Meisel RL. Knobil E, Neill JD, editors. The physiology of male sexual behavior. Physiology of reproduction. 1994. New York: Raven Press;3–105.
19. Allen LS, Hines M, Shryne JE, Gorski RA. Two sexually dimorphic cell groups in the human brain. J Neurosci. 1989. 9:497–506.
20. Kupfermann I. Kandel ER, Schwartz JH, Jessell TM, editors. Hypothalamus and limbic system: peptidergic neurons, homeostasis, and emotional behavior. Principles of neural science. 1991. Norwalk: Appleton and Lange;735–749.
21. Linas R, Ribary U, Contreras D, Pedroarena C. The neuronal basis for consciousness. Philos Trans R Soc Lond B Biol Sci. 1998. 353:1841–1849.
22. McGuire PK, Bench CJ, Frith CD, Marks IM, Frackowiak RSJ, Dolan RJ. Functional anatomy of obsessive-compulsive phenomena. Br J Psychiatry. 1994. 164:459–468.
23. Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behavior. Brain. 1995. 118:279–306.
24. Beauregard M, Karama S, Leroux JM, Lecours AR, Beaudoin G, Bourgouin P. The functional neuroanatomy of amusement, disgust and sexual arousal. 1998. In : Paper presented at the 4th International Conference on Functional Mapping of the Human Brain; Montreal, Quebec, Canada.
25. Lane RD, Reiman EM, Geoffrey LA, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. Am J psychiatry. 1997. 154:926–933.
26. Lane RD, Chua PM-L, Dolan RJ. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia. 1999. 37:989–997.
27. Buchel C, Josephs O, Rees G, Turner R, Frith CD, Friston KJ. The functional anatomy of attention to visual motion: a functional MRI study. Brain. 1998. 121:1281–1294.
28. Lane RD, Reiman EM, Bradley MM, et al. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997b. 35:1437–1444.
29. O'Craven KM, Rosen BR, Kwong KK, Treisman A, Savoy RL. Voluntary attention modulates fMRI activity in human MTMST. Neuron. 1997. 18:591–598.
30. Treue S, Maunsell JH. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996. 382:539–541.
31. Chawla D, Rees G, Friston KJ. The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci. 1999. 2:671–676.
32. Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color and speed: functional anatomy by positron. 1991.
33. Goldenberg G, Podreka I, Steiner M, et al. Contributions of occipital and temporal brain regions to visual and acoustic imagery; a SPECT study. Neuropsychologia. 1991. 29:695–702.
34. Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, et al. Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry. 1997. 154:918–925.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download