This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
To evaluate the fixation strength and tissue reaction of the glue fixation and self-stabilizing leg fixation methods and to compare the results with those of the conventional tagging suture fixation method.
Materials and Methods
Twelve healthy rabbits were selected and three different methods of implanting the port chamber were employed on the back of each rabbit. A total of thirty six port chambers were implanted with these three different methods, viz. the glue fixation method using tissue adhesive, the self-stabilizing leg method using a self-expandable stabilizing leg, and the suture fixation method. The fixation strength and the gross and histopathologic changes of each fixation method were evaluated at three days, one week, two weeks and four weeks after port implantation.
Results
The glue fixation method showed a good fixation strength, which was similar to that of the tagging suture method (p = 0.3486). Five of the six ports (83%) implanted with the glue fixation method which were examined after two weeks showed cracks on the external surface, but this had no adverse effects on their function. A large amount of granulation tissue reaction was found at the bottom of the chamber (p = 0.0025). The fixation with the self-stabilizing leg showed relatively lower fixation strength (p = 0.0043), but no turning-over of the chamber occurred. The fixation strength improved with time after the first week, and minimal granulation tissue reaction was observed with this method.
Conclusion
The glue fixation method exhibited equal fixation strength compared to the suture fixation, but showed cracking and a large amount of granulation tissue, whereas the fixation with a self-stabilizing leg showed weaker fixation strength.
Keywords: Catheters and catheterization, technology, Interventional procedures, comparative studies, Interventional procedures, experimental, Soft tissues
In totally implantable port catheter systems, a port catheter is first introduced within a vein or an artery, the catheter is connected with the port chamber, and then the chamber is inserted within the subcutaneous tissue and implanted (
1). After positioning the port catheter within the vein or artery, the chamber must be implanted in the subcutaneous tissue. To make this large enough, a subcutaneous pocket must be made through a skin incision of 4-5 cm in size and subcutaneous dissection, and for the fixation of the chamber within the pocket tunnel, a suture fixation is necessary, because there have been a few reports of migration of the ports occurring during the follow-up periods (
2,
3).
Therefore, for the subcutaneous dissection and fixation of the chamber, a surgical operation is needed. Also, because ligation deep within the subcutaneous pocket cannot easily be achieved, the skin incision and dissection becomes large, and sometimes the chamber cannot be positioned within the subcutaneous pocket, but has to be placed just below the skin incision area. Thus, when inserting an implantable port, there have been previous cases in which it was necessary to wait for the help of a surgeon or perform an unnecessarily large skin incision and subcutaneous dissection, in order to create the subcutaneous pocket (
4).
Many reports have described surgical procedures and radiologic procedures for the placement of port catheter systems (
5-
7). The major disadvantages of radiologic procedures are that complications, such as catheter dislodgement or hepatic arterial occlusion, are more frequently associated with these procedures than with surgical ones (
7). For this reason, several procedures have been developed for catheter tip fixation (
8-
10). Wacker et al. reported that catheter disconnection occurred between the therapy catheter and the tube to the infusion pump in 11 out of 33 patients who underwent catheter implantation with the subcutaneous transsubclavian approach (
11). In addition to the problem of the catheter tip fixation, the stability of the port chamber itself is another factor to consider, in order to prevent this type of complications from arising.
Therefore, in this report, the authors propose two new fixation methods designed to provide easier and more convenient chamber fixation, viz. the glue fixation method using tissue adhesive, and the self-stabilizing leg method using a self-expandable stabilizing leg. In an animal experiment, these two methods were compared with the existing suture fixation method, in terms of the fixation strengths, histopathologic changes around the port chamber and clinical usefulness.
MATERIALS AND METHODS
Twelve healthy New Zealand white rabbits were anesthetized by means of an intramuscular injection of 35 mg/kg of ketamine hydrochloride (Ketara®, Yuhan Corporation, Seoul, Korea) and 5 mg/kg of xylazine hydrochloride (Rompun®, Bayer Korea, Seoul). The hairs on the dorsal side of the rabbit were shaved using an electric razor. After disinfection, three port chambers, one with each of three different fixation methods, were implanted in the subcutaneous tissue on the back of each rabbit. A low profile 5.8 F Port-A-Cath (Sims-Deltec, St. Paul, Minn) was used.
The implanted areas used for each port on the rabbits' back were designated as follows: for the glue fixation method, the left upper quadrant was used, for the self-stabilizing leg method, the right upper quadrant was used, and for the suture fixation method, the right lower quadrant was used. The fixation strength for each fixation method and the gross and histopathologic changes were examined at three days, one week, two weeks and four weeks after implantation.
Glue Fixation Method
As the tissue adhesive glue, 0.5 cc of NBCA (N-butyl cyanoacrylate; Histoacyl, Braun, Melsugen, Germany) and 0.5 cc of Lipiodol (Gerbet, France) were mixed thoroughly in a polyethylene cup. This mixture was then aspirated using a 1 cc syringe without a needle for immediate use, because this adhesive glue starts to solidify right away, due to the polymerization reaction which occurs as soon as it comes into contact with blood or tissue. Therefore, this adhesive glue was prepared after the port chamber was securely inserted into the subcutaneous pocket on the rabbit's back. The adhesive glue in the 1 cc syringe was then immediately injected right underneath the port chamber.
In the left upper quadrant of the rabbit's back, about 2 cm of the skin was horizontally incised, so as to provide just enough room for the port chamber to enter. Following the subcutaneous dissection, a subcutaneous pocket was made on the rabbit's back. The port chamber was pushed into the subcutaneous pocket, inserted sufficiently deep (and more than 1 cm apart from the skin incision area), and then the tissue adhesive liquid was prepared. The lower surface of the chamber within the subcutaneous pocket was pulled up slightly, and then the 1 cc syringe containing the tissue adhesive liquid described above was inserted, in order for it to reach the deepest point of the chamber. Then the syringe was pulled slowly out while injecting the adhesive liquid, in order for the adhesive liquid to be injected evenly. About 0.5-0.7 cc of the liquid was injected. Upon the removal of the syringe, immediate adherence and fixation between the chamber surface and the subcutaneous pocket surface was observed. Care was taken to avoid any spillage of the adhesive liquid in the incised skin area. The subcutaneous tissue and skin were then sutured using silk stitch.
Self-stabilizing Leg Fixation Method
The self-stabilizing leg was made of nitinol wire attached to the bottom surface of the port chamber, and there were two legs for each port chamber. This was designed so that when inserting the chamber into the subcutaneous pocket through the skin incision, compressive insertion with the finger could be accomplished. In the subcutaneous pocket fixation position, the legs expand by themselves, due to the unique elasticity of nitinol, and this leads to their fixation with the surrounding tissues (
Fig. 1).
Nitinol wire was shaped into a metal model with a size and shape appropriate for each port chamber. The shaping of this wire into the self-stabilizing leg was done by applying heat with an automatic porcelain furnace with a vacuum program function, at 450℃ for twenty minutes, followed by rapid cooling in a coolant bath. Twelve pairs of stabilizing legs with identical shapes and sizes were made and attached to the bottom surface of the port chambers, and each port chamber system was then sterilized with ethylene oxide gas.
For the implantation of the ports with the self-stabilizing legs, after making a horizontal incision 2 cm in size and creating the necessary space for the port chamber by subcutaneous dissection in the right upper quadrant of the rabbit's back, the port chamber with the attached self-stabilizing legs was inserted sideways (horizontally), for easier insertion into the pocket, while compressing the two self-stabilizing legs with the fingers. After completing the insertion of the port system into the subcutaneous pocket, the orientation of the port system was adjusted vertically. The self-stabilizing leg expanded into its original two-leg shape by its own elasticity, settling into the subcutaneous layer. The position of the port chamber was then adjusted by pushing it with the fingers, followed by the closing of the subcutaneous layer and skin by means of a suture.
Suture Fixation Method
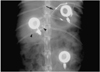
In the left lower quadrant of the rabbits' back, an approximately 3 cm long horizontal skin incision, which is longer than that used in the above two methods, was performed, and then subcutaneous dissection was performed from the upper area of the incision. The dissection was large enough to allow for stitch ligation through a fixating hole on the bottom surface of the chamber within the subcutaneous pocket. The port chamber was fixated more than 1 cm apart from the incised area on the skin, and then the subcutaneous tissue and skin were sutured. To confirm the position and shape of each port, a plain radiograph was taken (
Fig. 2).
Comparative Experiment Method
To determine the fixation strength of the port chambers, the degree of turning-over and degree of movement were evaluated. To induce the turning-over phenomenon, the port chambers implanted on the rabbits' backs were held with the fingers and then the port chamber was twisted by angles of 30°, 60° and 90° in each of 4 directions (top and bottom, left and right). In each case, it was observed whether the chambers turned over or not.
In addition, the fixation strength was evaluated by grading the movement into four levels. When the port chamber was held with the fingers and shaken to the left and right, up and down, if the distance moved was more than one half of the port's diameter, a grade of III was attributed. If the distance moved was between one half and one quarter of the port's diameter, a grade of II was assigned. If the distance moved was less than one quarter of the port's diameter, a grade of I was attributed. If there was no movement, a grade of 0 was attributed.
The positional changes and external changes of the port chamber in relation to the tissue adhesive and self-stabilizing legs were also evaluated.
The animals were sacrificed and then the port chambers and tissues around the chamber were removed all together. The gross and histopathologic changes of the tissues were examined by using a comparative grading system.
The degree of gross granulation tissue formation was graded as follows: grade 0 = no tissue change, grade I = the presence of granulation tissue formation in less than one half of the port chamber, grade II = between grade I and grade III and grade III = the presence of granulation tissue formation on the entire port chamber.
Microscopically, the degree of inflammatory change was determined and graded at 200 times magnification into four categories: grade 0 = less than 10 inflammatory cells, grade I = between 10 and 100 inflammatory cells, grade II = more than 100 inflammatory cells and grade III = microabscess.
The degree of microscopic granulation tissue formation was determined and graded: grade 0 = no granulation tissue formation, grade I = capillary proliferation, fibroblast proliferation with lots of inflammation cell infiltration but weak collagen infiltration, grade II = between grade I and III and grade III = notable collagen infiltration with rare capillary proliferation, fibroblast proliferation or inflammatory cell infiltration. No culture study was performed, because there was no discernible evidence of infection on gross inspection.
Each of the classified grades was analyzed using the split-plot design method. The software used for one-way analysis of variance (ANOVA) analysis was SAS (version 8.1), and the statistical significance was determined to be 5%.
RESULTS
A total of 36 port chambers were successfully implanted in 12 rabbits, and all of the rabbits were alive during the experimental period.
Comparison of Fixation Strengths of Different Port Chambers
No turning-over phenomenon occurred up to four weeks after implantation
On the third day after port implantation, the movement of the port chamber was evaluated as grade II for the glue and suture fixation methods (movement with a distance of between one half and one quarter of the port's diameter). However, the self-stabilizing leg method showed grade III movement (movement with a distance of more than one half of the port's diameter), exhibiting a relatively weaker fixation strength than the other two methods. After week 1, the fixation strength of the self-stabilizing leg method increased, but a comparison at weeks 2 and 4 showed persistently weaker fixation strength for this technique than for the other two fixation methods.
The glue fixation and suture fixation methods showed similar fixation strengths after port implantation throughout the study period (
Table 1).
Positional and External Changes of Port Chamber
After two weeks of port implantation, two of the three rabbits who were subjected to the self-stabilizing leg method showed a shifted position of the port chamber with a distance of one and half of the port diameter. In the case of the glue fixation method, one of the three rabbits also showed a shift of about one half of the port diameter. However, no such positional change occurred in the other two rabbits.
In the case of the glue fixation method, cracks on the plastic surface of the port chamber were seen from two weeks after implantation. However, there was no defect or malfunction in the silicon window or surrounding inner cavities. This phenomenon occurred in five of the six chambers implanted in the rabbits examined at weeks 2 and 4, while in the other rabbits, the adhesive and port were not in direct contact, due to abscess formation around the port chamber.
Comparison of Gross and Histopathologic Findings
The glue fixation method showed grade II gross granulation tissue formation from the third day after implantation, with glue remnants in the form of a white powder being observed on the bottom surface of the port which were easily detached (
Table 2). These remnants had the appearance of a hard mass, but this was localized on the bottom surface of the port chamber, without any further changes in the degree of tissue formation occurring as time progressed. Meanwhile, the self-stabilizing leg and suture fixation methods showed no granulation tissue formation up to one week after port implantation, but after two and four weeks following implantation, membranous granulation tissue was observed in the fixation hole on the bottom surface of the chamber. In a quantitative comparison of gross granulation tissue formation, the glue fixation method showed more granulation tissue formation over a wider area than the other two methods.
On histopathologic examination, microabscess formation was observed in two cases involving the glue fixation method at day 3 and week 1, respectively, however no abscesses were observed on gross inspection. In terms of the degree of inflammation (
Table 3) and granulation tissue formation (
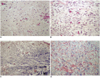
Table 4), at day 3, inflammatory responses such as capillary proliferation, fibroblast proliferation and inflammatory cell infiltration were observed. However, after one week, the degree of inflammation was relatively lower, while the degree of collagen infiltration showed a tendency to increase. There were no noticeable differences among the three groups even after four weeks, and there were no statistically significant differences either. In the Masson-Trichrome staining, areas with collagen fiber formation were seen as a purple color, which were easily recognizable. All of the groups showed increased staining as time progressed, due to collagen formation, but no significant differences were observed among the groups (
Fig. 3).
Statistical Significance Analysis
In the evaluation of the degree of movement for the purpose of comparing the fixation strengths of the port chambers between the different methods, the self-stabilizing leg method showed weaker fixation strength than the other two fixation methods (i.e., the glue fixation method and suture fixation method), and this difference was statistically significant (p = 0.0043). There was no significant difference in the fixation strengths between the glue fixation and suture fixation methods (p = 0.3486).
Gross examinations revealed more granulation tissue formation in the glue fixation method, with this difference being statistically significant (p = 0.0025), while there was no significant difference between the self-stabilizing leg and suture fixation methods (p = 0.2051). Histopathologic examinations showed no significant difference among the three groups in terms of the degree of inflammation or the degree of granulation tissue formation (p = 0.5060).
Removal of Port Chambers
In all three methods, the removal of the port chambers was successfully acomplished. Removal was easiest in the glue fixation method, whereas some difficulty was encountered in the suture fixation method.
DISCUSSION
The totally implantable port catheter system is a very convenient device for both the doctor and the patient when performed selectively on patients (
12-
14). The intravascular insertion of the port catheter, skin incision and subcutaneous dissection during the implantation of this device is a rather simple surgical maneuver; however, the process of fixating the port chamber within the subcutaneous pocket tunnel with ligation can be a highly complicated maneuver even for an expert surgeon. Moreover, in the case of insufficient fixation, the port chamber occasionally turns over. Rodgers et al. reported that the turning-over effect caused functional disorder in two out of 19 patients who showed complications (
2).
Because of these problems, the port chamber is usually fixated within the subcutaneous pocket using a tissue adhesive such as NBCA. When this method is clinically applied, the simple and quick fixation of the chamber implantation is possible, even with minimal skin incision and dissection and, in addition, a sufficient distance can be maintained between the skin incision and the chamber. However, no basic research has been reported on issues involving the existing method, such as a comparison of the fixation strength, the observation of histopathologic changes such as granulation tissue formation, and the problems arising from chemical and physical reactions.
This study showed that the glue fixation method was effective and provided high fixation strength. Although not officially evaluated or recorded in this research, the good fixation strength of this method was observed immediately following the injection of glue beneath the port chamber. When the fixation strength of the glue fixation method was evaluated at four weeks after implantation, it was found to be equivalent to that of the suture fixation method, with the result having statistical significance. Although the range of subcutaneous granulation tissue formation was significantly wider for the glue fixation method than for the other two fixation methods, the granulation tissues were localized in the bottom surface of the chamber and no further development of the granulation tissue was observed. Consequently, it is safe to say that this granulation tissue formation was localized in a position not affecting the silicon window and that it did not cause any difficulty in making the port window puncture for drug injection.
One more unique feature of the glue fixation method was the surface cracking of the port chamber that occurred two weeks after port implantation, although it neither hindered the silicon window puncture used for drug injection nor damaged the inner metal cavity. However, it is necessary take this problem into consideration and search for an external port chamber material that does not react with the tissue adhesive. No definite reason for this surface cracking has yet been elucidated; however it is presumed to result from a combination of chemical or physical reactions between the port chamber's external material and the tissue adhesive. This phenomenon was not observed in the first week after port implantation, but did occur in five out of the six rabbits examined at weeks 2 and 4 after implantation. Since there was no surface cracking at day 3 or after week 1, and there was no surface change at week 2 in the case where the chamber and granulation tissue were separated by abscess formation, it is assumed that the surface cracking was not caused by a chemical reaction alone.
Meanwhile, the port chambers used with the two-legged self-stabilizer were easily and conveniently implanted and fixated in the subcutaneous tissue in these animal experiments, and there was no difference in the installation time, with insertion being accomplished in a few seconds without difficulty. In terms of the fixation strength, the self-stabilizing method showed a significantly weaker strength than the other two methods, but, when applied clinically, there was no turning-over phenomenon which is a crucial advantage. Therefore, it could be concluded that the weaker fixation strength of this method does not result in its being less useful. The external changes, chemically and physically evident in the glue fixation method, were not evident in this method, affording it an additional advantage. When it comes to clinical application, however, the glue fixation method can be clinically applied immediately without the need for a new approval process, whereas the self-stabilizing leg method will require an approval process before it can be clinically applied, because of the use of a new apparatus.
The removal of the port chamber was easiest in the case of the glue fixation method, because granulation tissue was wrapped around the chamber like a capsule. After this part was dissected, the granulation tissue and chamber could be easily separated, allowing the removal process to be accomplished without difficulty. However, in the suture fixation method, the ligation stitches had to be located manually before they could be removed, and the granulation tissue which grew into the fixation hole before the separation process had to be dissected. The self-stabilizing leg method allowed easier and more convenient separation and removal than the suture fixation method.
The limitation of this study is that the stability of the port-catheter system does not depend solely on the port chamber fixation, and that the catheter tip fixation is more important in preventing catheter dislodgement or artery occlusion. To obtain more precise knowledge about the practical potential of the different fixation methods, a combined study is needed with the different catheter tip fixations.
In conclusion, in this study, we demonstrated the different characteristics, strengths and weakness of three port fixation methods by comparing their fixation strengths, the external changes of the ports, and the gross and histopathologic changes. The glue fixation method showed cracking and a large amount of granulation tissue, but equal fixation strength. The self-stabilizing leg fixation has relatively weaker fixation strength.








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download