Abstract
Objective
We wished to evaluate the effect of the Pringle maneuver (occlusion of both the hepatic artery and portal vein) on the pathologic changes in the hepatic vessels, bile ducts and liver parenchyma surrounding the ablation zone in rabbit livers.
Materials and Methods
Radiofrequency (RF) ablation zones were created in the livers of 24 rabbits in vivo by using a 50-W, 480-kHz monopolar RF generator and a 15-gauge expandable electrode with four sharp prongs for 7 mins. The tips of the electrodes were placed in the liver parenchyma near the porta hepatis with the distal 1 cm of their prongs deployed. Radiofrequency ablation was performed in the groups with (n=12 rabbits) and without (n=12 rabbits) the Pringle maneuver. Three animals of each group were sacrificed immediately, three days (the acute phase), seven days (the early subacute phase) and two weeks (the late subacute phase) after RF ablation. The ablation zones were excised and serial pathologic changes in the hepatic vessels, bile ducts and liver parenchyma surrounding the ablation zone were evaluated.
Results
With the Pringle maneuver, portal vein thrombosis was found in three cases (in the immediate [n=2] and acute phase [n=1]), bile duct dilatation adjacent to the ablation zone was found in one case (in the late subacute phase [n=1]), infarction adjacent to the ablation zone was found in three cases (in the early subacute [n=2] and late subacute [n=1] phases). None of the above changes was found in the livers ablated without the Pringle maneuver. On the microscopic findings, centrilobular congestion, sinusoidal congestion, sinusoidal platelet and neutrophilic adhesion, and hepatocyte vacuolar and ballooning changes in liver ablated with Pringle maneuver showed more significant changes than in those livers ablated without the Pringle maneuver (p < 0.05)
Conclusion
Radiofrequency ablation with the Pringle maneuver created more severe pathologic changes in the portal vein, bile ducts and liver parenchyma surrounding the ablation zone compared with RF ablation without the Pringle maneuver. Therefore, we suggest that RF ablation with the Pringle maneuver should be performed with great caution in order to avoid unwanted thermal injury.
Radiofrequency (RF) ablation is a local ablation technique designed to destroy tumors by heating the cancerous tissue (1). Numerous experimental and clinical studies have suggested that the technique is safe and effective in treating malignant hepatic tumors (2-13). For RF ablation to be completely successful, all the malignant tissue should be ablated. The strategy for successful RF ablation includes ablating an adequate margin, about 0.5-1 cm, of normal hepatic tissue surrounding the tumor as well as the entire tumor itself (14-18).
The hepatic inflow seriously limits the ablation zone size and alters its shape by creating heat sinks and carrying heat away from the area of ablation (19-21). The Pringle maneuver (occlusion of both the hepatic artery and portal vein) alleviated the heat sink effect associated with perfusion of the hepatic artery and portal vein and it increased the size of ablation zone (20, 22-26). However, the Pringle maneuver requires a laparotomy, which negates the minimal invasiveness advantage of RF ablation itself, and this invasiveness is an obvious disadvantage of the Pringle maneuver.
Since the test animals were sacrificed immediately after RF ablation in most of the previous animal studies (22-24, 26), any potential changes in hepatic vessels, bile ducts and liver parenchyma surrounding the ablation zone could not be assessed in those studies. Its been recently shown that RF ablation with vascular occlusion increased the risk of injury of surrounding biliary structures, portal vein or parenchyma (25), and RF ablation that comes in contact with the bile duct induced bile duct stenosis or dilatation (27). In those studies (25, 27), the pathologic changes in the hepatic vessels, bile ducts and liver parenchyma surrounding the ablation zone were not fully described.
The purpose of the present study was to evaluate the effect of the Pringle maneuver during RF ablation on the pathologic changes in the hepatic vessels, bile ducts and liver parenchyma surrounding the ablation zone in rabbit livers.
Our experimental protocol was approved by the Institutional Animal Care and Use Committee at our hospital. Twenty-four New Zealand white rabbits (all males and 2.6-3.3 kg each, mean weight: 3.0 kg) were randomly assigned to two treatment groups of 12 rabbits each: the control group and the experimental group with temporary occlusion of their portal veins and hepatic arteries (the Pringle maneuver). All the animals were handled and cared for in accordance with the recommendations of the National Research Council Guidelines for the Care and Use of Laboratory Animals.
All the rabbits were anesthetized by an intramuscular injection of 35 mg/kg ketamine hydrochloride (Ketamine; Yuhan, Seoul, Korea) and 5 mg/kg of xylazine (Rompun; Bayer Korea, Ansan, Korea). A 22-G x 1-inch IV catheter was inserted into the dorsal ear vein and the anesthesia was maintained with an intravenous injection of 17.5 mg/kg of ketamine hydrochloride every 40 minutes.
After an adequate state of anesthesia was achieved, the epigastrium and back of the rabbits were shaved and sterilized, and a grounding pad (13×21 cm) was applied to the rabbits' back. A midline laparotomy incision was performed from the xiphisternum to the umbilicus to expose the liver. The hepatic artery and portal vein were isolated by using umbilical tape, and they were temporarily occluded with an atraumatic vascular clamp.
Radiofrequency ablation was performed by using a 50-W, 480-kHz monopolar RF generator (model 500 series; Radiofrequency Interstitial Thermal Ablation Medical System, Mountain View, California) and an active expandable RF electrode (model 30; Radiofrequency Interstitial Thermal Ablation Medical System). The RF generator had instrument displays that indicated the hook temperatures, tissue impedance value, and treatment time. The RF electrode consisted of a 15-gauge shaft through which four sharp prongs, each 0.021 inches in diameter (25 gauge), could be deployed. When fully extended, the prongs were in an "umbrella" configuration, with each prong at a 90° interval and the entire "umbrella" device was 3 cm in diameter. The electrode was insulated with a 0.1-mm-thick plastic film and it had a noninsulated 1-cm distal part that delivered the RF current in addition to the four expandable electrodes. Each electrode had a thermistor at its tip to monitor the temperature in the surrounding tissue. In this study, the tip of the electrode was placed in the liver within 2 cm from the porta hepatis of the rabbit liver, and the distal 1 cm of the prongs was then deployed.
The RF generator was set at 50 W of power and applied for seven minutes in the mean-temperature-control mode with a target temperature of 100℃. This mode allowed the delivery of the maximum power output until the mean value of the temperatures measured at the tip of the four electrodes reached the threshold, whereon the generator maintained this mean temperature by automatically adjusting the power output. Hook temperatures, the tissue impedance value and treatment time were displayed on the panel of the RF generator during the procedure.
Radiofrequency ablation was performed with and without the Pringle maneuver in 12 rabbit livers. When Pringle maneuver was applied, the blood flow was restored to the liver immediately after the cessation of RF ablation.
Three rabbits of each group were sacrificed immediately, and then three rabbits were sacrificed at three days (the acute phase), seven days (the early subacute phase) and two weeks (the late subacute phase) after RF ablation by using an overdose of intravenous ketamine hydrochloride, and the livers were removed from the rabbits and examined.
Specimens of the livers were fixed in a 10% buffered formalin solution for a minimum of 24 hr for routine histologic processing. The formalin-fixed specimens were cut into 5-mm-thick slices at intervals perpendicular to the RF electrode tract, and they were macroscopically evaluated by measuring with calipers the maximum and minimum diameters of the ablation zone perpendicular to the electrode axis. Depth of ablation zone was measured along the electrode axis as the sum of the thickness of the axial sections. Based on previous reports (7, 27), we used the well-circumscribed central white zone seen on gross pathological examination to define the coagulation necrosis. The diameter of ablation zone was measured by consensus of two radiologists. Assuming that the ablation zones were spheroid, the volume was calculated by using the following formula:
Volume (cm3) = 4/3 π (maximum diameter/2) × (minimum diameter/2) × (depth/2)
The shape of ablation zone was characterized by the ratio between the maximum and minimum diameter.
These three dimensions, depth, maximum and minimum diameters and volume of the ablation zone, and the ratio between the maximum and minimum diameter were compared between the two groups with a Mann-Whitney U test. Significant statistical differences were defined as p values < 0.05.
The formalin-fixed slices of livers were evaluated by one of two pathologists for the gross changes in the hepatic vessels, bile ducts and the liver parenchyma surrounding the ablation zones.
Two tissue samples for each case were then embedded in paraffin, and 6-mm-thick sections were serially cut and stained with hematoxylin and eosin (H & E) and Masson trichrome stain. The other pathologist evaluated the following nine microscopic variables for changes in the hepatic vessels, bile ducts and liver parenchyma surrounding the ablation zone: portal inflammation, portal fibrosis, portal congestion, centrilobular necrosis, centrilobular congestion, sinusoidal congestion, sinusoidal platelet and neutrophilic adhesion, proliferation of bile duct epithelium, and hepatocyte vacuolar and ballooning changes. The grades of microscopic variables were rated according to the following four-point scale: 0, no change; 1, mild change; 2, moderate change; 3, severe change.
We compared the grades of microscopic variables between the two groups by using the Mann-Whitney U test. Statistically significant differences were defined as p values < 0.05.
The size in the three dimensions and the volume of the ablation zones created in rabbit livers with and without the Pringle maneuver are summarized in Table 1. The ablation zones created with the Pringle maneuver were significantly larger in all three dimensions and volumes than those ablation zones created without the Pringle maneuver: the mean maximum diameter was 2.8 cm versus 1.9 cm, the mean minimum diameter was 2.5 cm versus 1.8 cm, the mean depth was 2.2 cm versus 1.6 cm, and the mean volume was 8.7 cm3 versus 3.2 cm3, respectively, for the Pringle maneuver ablation zones versus the standard ablation zones (all the p values were < 0.05) (Fig. 1).
The ratio of between the maximum and minimum diameters for ablation zones created with the Pringle maneuver (1.21±0.16) was larger than that for the ablation zones created without the Pringle maneuver (1.08±0.05) (p = 0.08). The ratio between the maximum and minimum diameters for five ablation zones created with the Pringle maneuver was larger than 1.3, which represents an elliptical shape. The shapes of the RF ablation zones without the Pringle maneuver were near spherical with some distortion being noted from the heat sink effect created by the adjacent large blood vessels.
In the Pringle maneuver group, portal vein thrombosis was found in three cases (in the immediate phase [n=2] and acute phase [n=1]) (Fig. 2), bile duct dilatation adjacent to the ablation zone was found in one case (in the late subacute phase [n=1]) (Fig. 3), and infarction adjacent to the ablation zone was found in three cases (in the early subacute phase [n=2] and late subacute [n=1] phase) (Fig. 4). None of the above changes was observed in the livers ablated without the Pringle maneuver.
The mean grades of the microscopic variables surrounding the ablation zones created in the rabbit livers with and without the Pringle maneuver are summarized in Table 2. Centrilobular congestion, sinusoidal congestion, sinusoidal platelet and neutrophilic adhesion, and hepatocyte vacuolar and ballooning changes in livers ablated with the Pringle maneuver showed more significant changes than those changes observed in livers ablated without the Pringle maneuver (p < 0.05) (Fig. 5). The bile duct epithelium was mildly proliferated in two cases of livers ablated with the Pringle maneuver (Fig. 6), while the bile duct epithelium was normal in livers ablated without the Pringle maneuver. The mean grade of centrilobular necrosis between the two groups was similar, but the extent of centrilobular necrosis in livers ablated with the Pringle maneuver was significantly larger than that in the livers ablated without the Pringle maneuver (p < 0.05).
The mean grades of the microscopic variables surrounding the ablation zones created in the rabbit livers with the Pringle maneuver in each phase are summarized in Table 3. Portal congestion in livers ablated with the Pringle maneuver at the immediate and acute phases showed more significant changes than that in those livers at the early and late subacute phases (Fig. 7). Centrilobular necrosis in livers ablated with the Pringle maneuver at the early and late subacute phases showed more significant changes than the changes in those livers at the immediate and acute phase.
The goal of RF ablation is to consistently produce a zone of necrosis large enough to encompass the hepatic tumor with an appropriate safety margin of ablated normal tissue. The size of the resulting necrosis is determined by a variety of factors, including the electrode size and gauge, ablation temperature and duration, and these factors have been extensively studied (28). An adequate ablative margin of at least 0.5 cm of normal hepatic tissue surrounding the tumor is ideal to decrease the residual unablated tumor or local tumor progression (14-18).
The Pringle maneuver, which is a temporal occlusion of the hepatic artery and portal vein, can be obtained by compressing the hepatoduodenal ligament with the fingers or by a vascular clamp. The safe hepatic ischemic time is difficult to determine under the variable conditions of shock, hypovolemia and hypothermia. Therefore, periodic release of the occluded portal triad is best done every 15 to 30 minutes to allow for hepatic perfusion (29).
The hepatic inflow seriously limits the ablation zone size and it can alter the shape of the ablation zone due to the heat sink effect of blood flow (20, 21). Several investigators have claimed that the size of the ablation zone can be increased by means of occlusion of hepatic inflow (20, 22-26). Our investigation of the Pringle maneuver also revealed that the ablation zones created with the Pringle maneuver were significantly larger on all three dimensions and volume than those ablation zones created without the Pringle maneuver. The Pringle maneuver helps overcome the heat sink effect of tissue perfusion and thereby increases the size of ablation zone (20, 22-26). Compared to RF ablations performed with normal perfusion, RF ablations performed with occlusion of the hepatic inflow, such as with using the Pringle maneuver, have superior size and shape, and this improvement for the ablation geometry translates into greater tumor-free margins. In our study, five ablation zones created with the Pringle maneuver were elliptical shaped and these shapes may have been due to the surrounding infarction or the zones' close proximity to the liver surface.
The combination of RF ablation and hepatic artery occlusion would seem feasible to achieve in a clinical setting (30). Temporary occlusion of the hepatic artery can be accomplished by balloon occlusion during RF ablation. Hepatic artery occlusion can also be accomplished with selective transarterial chemoembolization before RF ablation, which would have the added benefit of treating the tumor with both heat and high concentrations of chemotherapy. To embolize or inflate a balloon in the hepatic artery is simpler and less prone to complication than to introduce a balloon in the portal vein through a transhepatic approach.
When used for short periods of time, the Pringle maneuver is considered to be safe during hepatic resection (31, 32). Blood inflow protects the integrity of the vascular and biliary structures during RF ablation, and the Pringle maneuver combined with RF ablation may result in injuries to the blood vessels or bile ducts (20, 31, 32). Two cases of vascular injury have been reported following intraoperative RF ablation with the Pringle maneuver (33, 34). On the other hand, Curley et al. (8) found no vascular or biliary injuries in 92 patients who underwent intraoperative RF ablation using the Pringle maneuver (median follow-up: 15 months). Any direct thermal injuries may affect the vascular or biliary structures adjacent to the ablation zone. Therefore, RF ablation should be performed with caution in treating the tumors near the major vascular and biliary structures.
In a recent animal study (25), biliary stenosis was found in four cases (two arterial occlusions, one portal occlusion, and one arterioportal occlusion). In another recent animal study (27), biliary stenosis, with or without upstream bile duct dilatation, or complete interruption of the bile duct were observed with the use of RF ablation. In our study, bile duct dilatation adjacent to the ablation zone was found in one case that was ablated with the Pringle maneuver. Because we did not perform ex-vivo cholangiograms using direct intubation of the main bile duct, the bile duct changes may have been underestimated compared with the above previous study (25). On microscopic examination, mild proliferation of bile duct epithelium was found in two cases of livers ablated with the Pringle maneuver (Fig. 5), while the bile duct epithelium was normal in livers ablated without the Pringle maneuver. Portal tract damage such as occlusion of the hepatic inflow can induce proliferation of the duct epithelial cells and also looping and reduplication of bile ducts (35).
Two cases of portal vein thrombosis have been reported following intraoperative RF ablation with the Pringle maneuver (32, 33). In a recent animal study (25), partial portal vein thrombosis adjacent to the ablation zone was found via ultrasound in one case of portal occlusion during RF ablation and this resolved two weeks after ablation. Our study also showed similar results with portal vein thrombosis being found in three cases using the Pringle maneuver (in the immediate [n=2] and in the acute phase [n=1]) whereas no portal vein thrombosis was found in the livers ablated without the Pringle maneuver. Moreover, none was found in livers at the early and late subacute phases. On microscopic examination, portal congestion in the livers ablated with the Pringle maneuver at the immediate and acute phases showed more significant changes than that in those livers at the early and late subacute phases. These results may suggest that occlusion of hepatic inflow may cause portal vein thrombosis at the immediate and acute phase, but the later continuous blood flow to the portal vein may wash out the thrombosis.
In a recent animal study (25), ischemic damage of the liver parenchyma adjacent to the ablation zone was found in two cases using arterioportal occlusion. The liver surface peripheral to the ablation zone displayed a pale, wedge-shaped area starting from the ablation zone to the periphery of the liver. Our study also showed similar results: infarctions in the liver adjacent to the ablation zones were found in three cases with the Pringle maneuver (in the early subacute [n=2] and late subacute [n=1] phases) (Fig. 4). These infarctions may have been due to combination of ischemia and heating, and they may become apparent during the subacute phase. On microscopic examination, this type of infarction exhibited ischemic coagulative necrosis of the hepatocytes with loss of nuclei and preservation of the general tissue architecture (Fig. 4B).
On microscopic examination, centrilobular and sinusoidal congestion were more severe in the livers ablated with the Pringle maneuver. These results may suggest that RF ablation with the Pringle maneuver causes more severe microvascular change or injury. Sinusoidal platelet and neutrophilic adhesion represents the inflammatory reaction and more severe inflammatory reaction was seen in livers ablated with the Pringle maneuver. Hepatocyte vacuolar and ballooning changes represent the damage from toxic insult such as thermal injury, and more severe damage was seen in livers ablated with the Pringle maneuver (36).
Occlusion of hepatic inflow, as during the Pringle maneuver, may not be necessary for RF ablations of all liver tumors, but it is a possible option for treating large or highly vascular tumors or those tumors in close proximity to the large blood vessels. However, RF ablation combined with the Pringle maneuver should be performed with caution because any direct thermal injuries may affect the vascular or biliary structures, or the liver parenchyma adjacent to the ablation zone.
Our study had several limitations. First, because we did not use imaging guidance for the RF ablation, the exact placement of electrodes in the same area of the liver near the porta hepatis was impossible, although we tried to place the tip of the electrode into the liver within 2 cm from the porta hepatis.
Second, rabbit livers are small and so we restricted the power and time of the RF energy to a lower level as compared to the true human clinical parameters (2-13). Therefore, the effect of the Pringle maneuver during RF ablation on the pathologic changes in the hepatic vessels, bile ducts and liver parenchyma surrounding the ablation zone might have been underestimated.
Third, all the recent RF devices use more powerful generators capable of producing 150-200 W, whereas we used a 50-W generator, which might not have been strong enough to overcome cooling of the perfused tissue gaps between the prongs (22) and therefore, this may have produced less effect on hepatic vessels, bile ducts and liver parenchyma surrounding the ablation zone. More changes in these structures may have been observed if we had performed the study with a high power generator.
Fourth, because we performed neither a direct cholangiogram nor a portogram, the changes in the portal vein and bile ducts might have been underestimated.
In conclusion, the Pringle maneuver used during RF ablation demonstrated more severe pathologic changes in the portal veins and bile ducts, and also more severe parenchymal changes surrounding the ablation zone. Therefore, although further experimental and clinical studies are needed for confirmation, RF ablation with the Pringle maneuver should be performed with great caution to avoid the unwanted thermal damage to blood vessels and bile ducts, and especially performing ablation with more powerful generators and larger electrodes.
Figures and Tables
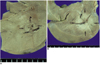 | Fig. 1Photographs of gross specimens of rabbit livers resected immediately after radiofrequency ablation.
A. Liver ablated without the Pringle maneuver.
B. Liver ablated with the Pringle maneuver. The ablation zone (arrows) created
with the Pringle maneuver (B) is substantially larger than that (arrows) of the
A liver ablated without the Pringle maneuver (A).
|
 | Fig. 2Photograph of a gross specimen of a rabbit liver resected three days (in the acute phase) after radiofrequency ablation with the Pringle maneuver. The dissected liver shows portal vein thrombosis (thin arrows) adjacent to the ablation zone (arrows). |
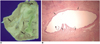 | Fig. 3Gross specimen (A) and microphotograph (B) of a rabbit liver resected two weeks (in the late subacute phase) after radiofrequency ablation with the Pringle maneuver.
A. Gross specimen shows tortuous dilatation of the bile duct (thin arrows) adjacent to the ablation zone (arrows).
B. Microphotograph (H & E, ×40) shows the markedly dilated bile duct (arrows).
|
 | Fig. 4Fresh gross specimen (A) and microphotograph (B) of a rabbit liver resected seven days (in the early subacute phase) after radiofrequency ablation with the Pringle maneuver.
A. Fresh gross specimen shows a wedge shape infarction (thin arrows) adjacent to the ablation zone (arrows).
B. Microphotograph (H & E, ×100) of the infarction exhibits coagulative necrosis of the hepatocytes with loss of nuclei and preservation of the general tissue architecture.
|
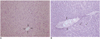 | Fig. 5Microphotographs (H & E, ×100) of the resected specimens obtained immediately after radiofrequency ablation. The microphotograph of the specimen ablated with the Pringle maneuver (B) shows the distended central vein (arrows) and sinusoids (thin arrows), suggesting congestion, while the central vein (arrows) and sinusoids are normal in the liver ablated without the Pringle maneuver (A). |
 | Fig. 6Microphotographs (H & E, ×400) of resected specimens obtained immediately after radiofrequency ablation. The microphotograph of the liver specimen ablated with the Pringle maneuver (B) shows mild proliferation of bile duct epithelium (arrows), whereas it is normal (arrows) in the liver ablated without the Pringle maneuver (A). |
 | Fig. 7Microphotographs (H & E, ×100) of resected specimens obtained after radiofrequency ablation with the Pringle maneuver. The microphotograph of the resected specimen obtained immediately after ablation (A) shows the distended portal vein (arrows), suggesting congestion, while the portal vein (arrows) is normal in the specimen obtained two weeks after ablation (B). |
Table 1
Three Dimensions and Volume of Ablation Zones Created in Rabbit Livers with and without Pringle Maneuver

References
1. McGhana JP, Brock JM, Tesluk H, Gu WZ, Schneider P, Browning PD. Hepatic ablation with use of radio-frequency electrocautery in the animal model. J Vasc Interv Radiol. 1992. 3:291–297.
2. Rossi S, Di Stasi M, Buscarini E, et al. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996. 167:759–768.
3. Solbiati L, Ierace T, Goldberg SN, et al. Percutaneous US-guided radio-frequency tissue ablation of liver metastases: treatment and follow-up in 16 patients. Radiology. 1997. 202:195–203.
4. Livraghi T, Goldberg SN, Monti F, et al. Saline-enhanced radiofrequency tissue ablation in the treatment of liver metastases. Radiology. 1997. 202:205–210.
5. Solbiati L, Goldberg SN, Ierace T, et al. Hepatic metastases: percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology. 1997. 205:367–373.
6. Goldberg SN, Gazelle GS, Solbiati L, et al. Ablation of liver tumors using percutaneous RF therapy. AJR Am J Roentgenol. 1998. 170:1023–1028.
7. Goldberg SN, Walovitch RC, Straub JA, Shore MT, Gazelle GS. Radio-frequency-induced coagulation necrosis in rabbits: immediate detection at US with a synthetic microsphere contrast agent. Radiology. 1999. 213:438–444.
8. Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999. 230:1–8.
9. Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radiofrequency ablation of medium and large lesions. Radiology. 2000. 214:761–768.
10. Rossi S, Buscarini E, Garbagnati F, et al. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol. 1998. 170:1015–1022.
11. Sironi S, Livraghi T, Meloni F, De Cobelli F, Ferrero C, Del Maschio A. Small hepatocellular carcinoma treated with percutaneous RF ablation: MR imaging follow-up. AJR Am J Roentgenol. 1999. 173:1225–1229.
12. Solbiati L, Goldberg SN, Ierace T, Dellanoce M, Livraghi T, Gazelle GS. Radio-frequency ablation of hepatic metastases: postprocedural assessment with a US microbubble contrast agent-early experience. Radiology. 1999. 211:643–649.
13. Lim HK, Choi D, Lee WJ, et al. Hepatocellular carcinoma treated with radiofrequency ablation: evaluation with longterm follow-up multiphase helical CT. Radiology. 2001. 221:447–454.
14. McGhana JP, Dodd GD 3rd. Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001. 176:3–16.
15. Lim HK. Radiofrequency thermal ablation of hepatocellular carcinomas. Korean J Radiol. 2000. 1:175–184.
16. Dodd GD 3rd, Soulen MC, Kane RA, et al. Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. RadioGraphics. 2000. 20:9–27.
17. Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000. 174:323–331.
18. Rhim H, Goldberg SN, Dodd GD 3rd, et al. Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. RadioGraphics. 2001. 21:S17–S35.
19. Gilliams AR, Lees WR. The importance of large vessel proximity in thermal ablation of liver tumors (abstr). Radiology. 1999. 213(P):123.
20. Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998. 227:559–565.
21. Rossi S, Garbagnati F, De Francesco I, et al. Relationship between the shape and size of radiofrequency induced thermal lesions and hepatic vascularization. Tumori. 1999. 85:128–132.
22. Chinn SB, Lee FT Jr, Kennedy GD, et al. Effect of vascular occlusion on radiofrequency ablation of the liver: results in a porcine model. AJR Am J Roentgenol. 2001. 176:789–795.
23. Chang CK, Hendy MP, Smith JM, Recht MH, Welling RE. Radiofrequency ablation of the porcine liver with complete hepatic vascular occlusion. Ann Surg Oncol. 2002. 9:594–598.
24. Scott DJ, Fleming JB, Watumull LM, Lindberg G, Tesfay ST, Jones DB. The effect of hepatic inflow occlusion on laparoscopic radiofrequency ablation using simulated tumors. Surg Endosc. 2002. 16:1286–1291.
25. Denys AL, De Baere T, Mahe C, et al. Radio-frequency tissue ablation of the liver: effects of vascular occlusion on lesion diameter and biliary and portal damages in a pig model. Eur Radiol. 2001. 11:2102–2108.
26. Aschoff AJ, Merkle EM, Wong V, et al. How does alteration of hepatic blood flow affect liver perfusion and radiofrequency-induced thermal lesion size in rabbit liver? J Magn Reson Imaging. 2001. 13:57–63.
27. Marchal F, Elias D, Rauch P, Leroux A, et al. Biliary lesions during radiofrequency ablation in liver. Study on the pig. Eur Surg Res. 2004. 36:88–94.
28. Raman SS, Lu DS, Vodopich DJ, Sayre J, Lassman C. Creation of radiofrequency lesions in a porcine model: correlation with sonography, CT, and histopathology. AJR Am J Roentgenol. 2000. 175:1253–1258.
29. Townsend CM, Beauchamp DR, Evers BM, Mattox KL. Hoyt DB, Coimbra R, Winchell RJ, editors. Sabiston textbook of surgery: the biological basis of modern surgical practice. Management of acute trauma. 2001. Philadelphia: WB Saunders;337–338.
30. Rossi S, Garbagnati F, Lencioni R, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000. 217:119–126.
31. Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997. 226:704–711.
32. Man K, Fan S, Ng IO, et al. Tolerance of the liver to intermittent Pringle maneuver in hepatectomy for liver tumors. Arch Surg. 1999. 134:533–539.
33. Jiao LR, Hansen PD, Havlik R, Mitry RR, Pignatelli M, Habib N. Clinical short-term results of radiofrequency ablation in primary and secondary liver tumors. Am J Surg. 1999. 177:303–306.
34. Scudamore CH, Lee SI, Patterson EJ, et al. Radiofrequency ablation followed by resection of malignant liver tumors. Am J Surg. 1999. 177:411–417.
35. Crawford JM. Kumar V, Abbas AK, Fausto N, editors. Liver and biliary tract. Robbins and Cotran pathologic basis of disease. 2004. 7th ed. Philadelphia: Elsevier Saunders;921.
36. Crawford JM. Kumar V, Abbas AK, Fausto N, editors. Liver and biliary tract. Robbins and Cotran pathologic basis of disease. 2004. 7th ed. Philadelphia: Elsevier Saunders;880–881.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download