Abstract
Objective
To assess the follow-up results after negative findings on unenhanced hepatic MR imaging in rectal cancer patients who have undergone locally curative surgery.
Materials and Methods
From all pertinent imaging reports and medical records, we selected 255 patients who had negative results on unenhanced hepatic MR imaging. When selecting patients who had undergone curative resection, the following patients were excluded from the study: 1) patients in whom extrahepatic metastases were detected on preoperative staging work-ups, 2) patients in whom the surgery was judged to be non-curative due to peritoneal seeding or local aggressiveness. Cases with follow-up periods of less than 18 months were also excluded, as these cases were considered insufficient to confirm the negative outcomes. Thus, a total of 149 patients were ultimately enrolled in our study. The follow-up results of unenhanced MR imagings were assessed according to the assumption that the newly developed hepatic metastases had been false-negative lesions on preoperative MR image.
Hepatic metastasis is a prevalent cause of death, even in patients undergoing apparently curative resections for colorectal cancer (1). Previous studies have shown that the majority of these patients harbor occult liver metastases, i.e. those which are undetectable by the surgeon upon laparotomy, or on conventional pre-operative imaging (2, 3). As surgical resection may offer a better survival rate and quality of life (4-6), an accurate preoperative assessment of the presence and distribution of hepatic metastases is vital.
In several previous studies (7, 8), surgical palpation, along with intraoperative ultrasonography (IOUS) findings, is regarded as the gold standard for the assessments of accuracy in preoperative imaging modalities. However, this type of analysis does not take into account any undetected liver metastases during surgery. True accuracy can be assessed only after a sufficient amount of time has elapsed, in order to allow the occult hepatic metastases to grow to a size sufficient for detection (9).
MR imaging has been used increasingly for preoperative local staging of rectal carcinomas (10-12). In our institution, unenhanced hepatic MR imaging is routinely incorporated into a preoperative MR examination for the purpose of providing an all-in-one staging procedure. Using this protocol, contrast-enhanced hepatic MR imaging, or IOUS, is performed only when a hepatic resection is being considered based on the findings of unenhanced hepatic MR imaging. IOUS has been advocated as the most accurate method for the diagnosis of liver metastasis (13-15). Furthermore, some authors have advocated the routine use of IOUS for the screening of liver metastases, even in patients with negative findings on preoperative imaging studies (16, 17). However, IOUS necessitates the wide surgical exposure of the liver for accurate evaluation. Therefore, a preoperative screening method with a high negative predictive value is necessary to preclude unnecessary IOUS examinations. Our anecdotal experience with negative findings on preoperative MR imaging in patients who had undergone curative surgery suggests that the incidence of hepatic metastasis on follow-up CT is relatively low.
Therefore, this study assessed the follow-up results after negative findings on unenhanced hepatic MR imaging in patients who had undergone curative surgery for rectal carcinoma.
Between February 1998 and March 2001, a total of 286 consecutive patients with histopathologically proven rectal adenocarcinoma underwent preoperative MR imaging for the local staging of tumors, as well as for the detection of hepatic metastases.
One investigator (J.S.L.) reviewed all the pertinent imaging reports and medical records. From these, we selected 255 patients who had scored negative results on unenhanced hepatic MR, excluding patients with lesions reported as potentially representing hepatic metastasis on preoperative MR reports (n = 31). In addition, the following patients were excluded from the study: 1) patients in whom the extrahepatic (n = 3, lung or retroperitoneal lymph node) metastases were detected by preoperative staging work-up (physical exam, measurement of carcinoembryonic antigen, chest roentgenogram, and MRI), 2) patients in whom the surgery was judged to be non-curative (n = 13) due to peritoneal seeding or local aggressiveness. These 16 patients were excluded from our analysis because, although a hepatic metastasis may develop in these patients on follow-up studies, it cannot be determined that the patients harbored undetected occult metastases (false negative) upon preoperative MR imaging. The remaining 239 patients were considered for inclusion in the study if they had the appropriate follow-up. Seven patients were entirely lost to follow-up. Eighty-three patients had postoperative CT follow-ups at less than 18 months, and were excluded as it was not appropriate to dismiss the possibility of a very slowly growing metastasis with this relatively short-term follow up period. The final study group consisted of 149 patients. Ninety-seven men and 52 women were included in the study, with a mean age of 59.2 years (range, 22 to 85 years). In all cases, surgery was performed by the same surgeon (N.K.K). The surgeon intraoperatively palpated the liver in all patients. The histological stages of the selected patients are shown in Table 1, according to the TNM system.
The time interval between MR imaging and operation was 1-34 days (mean, 8 days). The MR imaging was performed with a 1.5-T MR scanner (Signa Horizon; GE Medical Systems, Milwaukee, Wis). All images were obtained in the axial plane, using a phased-array multicoil. A rectangular field of view of 32×24-29×22 cm, which was adjusted for each patient, was held constant for all sequences. The MR imaging consisted of a) a respiratory-triggered T2-weighted fast spin-echo (FSE) (effective TR range/effective TE of 3500-10900/96-105, echo train length of 8-16, two signal averages, a matrix of 256×256, chemical shift fat suppression, superior and inferior spatial pre-saturation, chemically selective fat saturation, and a 7-8-mm slice thickness with a 1-2-mm gap), b) a breath-hold T1-weighted fast multiplanar spoiled gradient recalled-echo (GRE) in-phase (150-200/4.2-4.4, flip angle of 90°, one signal acquired, a matrix of 256×128, 8-10-mm slice thickness, and zero gap, interleaved), and c) a breath-hold T2-weighted single-shot half-Fourier image (effective TE 180, a matrix of 256×160, and a 7-8-mm slice thickness with a 1-2-mm gap). Contrastenhanced studies were performed only when a patient was suspected of hepatic metastasis on the unenhanced studies, and no such patients were included in this study.
Postoperative follow-up tests were routinely prescribed for the first two years in our institution. These tests included a physical examination, measurements of carcinoembryonic antigen levels, liver function tests every three months, and CT imaging every six months.
The CT scans were performed using a single-detector CT (Somatom plus-S [Siemens, Erlangen], HiSpeed CT/I [GE Medical Systems, Milwaukee, Wis]) or multidetector CT (Lightspeed Plus [GE Medical Systems, Milwaukee, Wis]) with a contiguous 5-8 mm slice thickness and a pitch of 1-1.5. A 60% wt/vol contrast medium (approximately a 90% higher osmolarity, and 10% lower osmolarity agent) was administered intravenously at a rate of 2-4 mL/sec, using an automatic power injector with a volume of 2 mL/kg, up to a maximum volume of 150 ml. CT scans were obtained approximately 70 seconds (parenchymal phase) after the initiation of contrast material injection.
The final diagnosis of the presence or absence of a liver metastasis was made as follows: 1) When a new liver tumor appeared on the follow-up CT, the final diagnosis of the liver metastasis was made histologically via surgical resection, clinically by typical appearance on the CT and/or additional MR images, or the demonstration of growth on the further follow-up CT, 2) The patients were considered to be free of occult hepatic metastases if no liver lesions had developed for at least 18 months after surgery, 3) The final diagnosis of liver metastasis was based on the latest imaging findings when a patient had died during the follow-up period. In addition, the patients who died without hepatic metastasis on follow-up CT after less than 18 months were excluded, according to our patient exclusion criteria (listed above).
Metastatic lesions diagnosed during the follow-up period were considered to be false-negative lesions on preoperative MRI. The negative predictive value was defined as the number of patients without metastases on the follow-up CT, divided by the number of patients without metastases on the preoperative MRI. However, in order to determine that they were truly new or missed on the preoperative MRI, the MR images were retrospectively reviewed, then compared with the follow-up CT by consensus of the two reviewers (J.S.L. and J.H.K.). Consensus was achieved when both reviewers agreed that a metastatic nodule was present or or absent on a preoperative MRI.
No unexpected hepatic metastases were found in any of the patients included in the analysis by palpation. The mean follow-up period was 29.4 months after surgery. The median follow-up period was 29.3 months, and the longest interval was 51.3 months. During the follow-up period, 106 (73%) patients received chemotherapy (n = 18) or chemoradiation (n = 88). At the end of this study, 23 (15.4%) of the 149 patients developed recurrences of the tumor (local recurrence without a remote recurrence in four patients, remote recurrence without a local recurrence in 16, and a remote and local recurrence in three patients). There were 10 mortalities over the postoperative follow-up period. Four patients died from causes not related to rectal cancer. The spread of the rectal cancer constituted the cause of death in the other 6 patients. The survival rates were 98.7%, 95.6%, 91.8%, and 84.8%, with follow-up times of 12, 24, 36, and 48 months (median, 29.3 months), respectively (Fig. 1).
Twenty-five hepatic metastatic lesions appeared during the follow-up period in 13 patients (8.7%). The histologic stages of those patients are as follows: Tumor invasion of the muscularis propria without nodal metastasis in three patients (T2, N0), tumor invasion into the perirectal tissues without nodal metastasis in three patients (T3, N0), tumor invasion into adjacent structures without nodal metastasis (T4, N0) in one patient, one to three local lymph nodes involved in one patients regardless of the depth of the tumor invasion (Any T, N1), and four or more pericolic lymph nodes involved in five patients, regardless of the depth of the tumor invasion (Any T, N2). The interval between surgery and the diagnosis of hepatic metastases ranged from 2.6 to 28.2 months (mean: 11.6 months). The tumors from 11 patients were detected before 18 months, while the tumors from the remaining two patients were detected after 21.3 months and 39 months, respectively. Among the 13 patients, five exhibited solitary metastatic tumors, while eight patients had multiple tumors at the time of the initial diagnosis of the hepatic metastases: two tumors in five patients, three tumors in two patients, and four tumors in one patient. These 25 metastatic lesions were not observed on the preoperative MR images in consensus interpretation, retrospectively, although the follow-up CT images were compared.
On the follow-up CT findings of the tumors in the 13 cases, 11 patients exhibited unequivocal findings of hepatic metastasis. In two cases, small lesions of less than 1 cm were judged to be metastatic lesions only after these lesions showed growth on serial CT scans (Fig. 1). Histologic confirmation was obtained by surgical resection in four out of 13 patients. In the remaining patients, the clinical diagnosis of hepatic metastasis was made according to serial CT findings (typical appearance of a metastatic tumor or growth in size on the serial studies) as well as laboratory findings, including elevations in carcinoembryonic antigen levels. The metastases in these 13 patients were regarded to be present at the time of surgery, but were not identified by preoperative MR images. Therefore, the tumors in these 13 patients (8.7%) were judged to be false negatives. The calculated negative predictive values were 91.3% (136 out of 149 patients).
Intraoperative ultrasonography is believed to be the most sensitive imaging technique for the detection of hepatic metastases (13-15). Conlon et al. suggested that IOUS provides the most useful additional information with regard to hepatic lesions, despite recent improvements in preoperative MRI (18). However, in order to examine the entire liver by IOUS, the liver needs to be mobilized, and its diagnostic accuracy is then operator-dependent. Therefore, the routine use of IOUS in all patients who have undergone colorectal surgery is practically impossible. The goal of this study was to determine whether noninvasive MR imaging could be used as a selective method, to preclude unnecessary IOUS examinations.
Some studies have reported false negative results from IOUS on patients undergoing apparently curative surgeries (8, 9, 19). Leen et al. (9) reported that 22 patients developed overt liver metastases after a two-year follow-up in 87 patients with negative findings on IOUS, in the evaluation of hepatic metastasis (the negative predictive value based on the follow-up result: 74.7%). The percentage of false negative IOUS results (number of patients with metastasis on follow-up / number of patients with negative findings on IOUS) was reported to be 9.0% (13/144) at 37 months by Machi et al. (8) and 13% (6/47) at 23 months by Stone et al. (19). In this study, the overall false-negative result for MR imaging was 8.7% after 29.4 months, indicating an overall negative predictive value of 91.3%. The negative predictive value for MR imaging in this study was comparable to that obtained via IOUS.
Several limitations were inherent in this study. Although newly discovered lesions on CT were defined as occult metastases, other explanations might be adopted to rationalize the existence of these lesions. The liver metastases which were discovered later may have occurred as the result of primary rectal cancer manipulation during surgery. They might also have occurred postoperatively, from primary cancer which had been partially left behind, due to an incomplete resection. It is believed that these possibilities were excluded due to our selection of only those patients whose pathological specimens exhibited a tumor-free resection margin. Secondly, advanced cases which required treatment by postoperative chemotherapy comprised the majority (115 patients [77%] were more than T2N0) of this study. Among these advanced cases, 106 patients received postoperative adjuvant systemic chemotherapy (71.1% among the 149 patients of the study group). Therefore, the metastatic lesions could have been eradicated, or at least suppressed, by the chemotherapy, thereby resulting in higher negative predictive value for the preoperative MR imaging. If there had been some metastatic lesions which could have been eradicated by chemotherapy, they might not need to have been resected at the time of surgery. Therefore, this would not affect the significance of the preoperative MR imaging. Thirdly, our follow-up criteria (18 months) may have been insufficient for the confirmation of the truly negative cases. It has been reported that 80% of all recurrence develops during the first two years after curative resection of colorectal cancer (20, 21). Thus, it may be inappropriate to dismiss the possibility of very slowly growing metastases within the relatively short-term follow up period. More extended follow-up results may be necessary to establish more reasonable negative predictive values. Finally, no contrast enhancement study was performed in this work. Other studies have emphasized the importance of dynamic gadolinium-enhanced MR for the characterization of focal hepatic lesions (22-24). Several studies have also reported that liver MRI using gadobenate dimeglumine or ferumoxides increased the detection of liver lesions, particularly in cases of primary hepatic neoplasm (25, 26). However, controversy remains with regard to the utility of gadolinium-enhanced MR images for the improvement of the detection rate of liver metastasis. In some studies, the detection of non-hepatocellular carcinomas (including metastases) did not increase due to the taking of dynamic images (27, 28). Currently, tissue-specific contrast agents, including superparamagnetic iron oxides and manganese agents have been used to improve the detection of hepatic metastases. However, the clinical indications for these agents have not yet been established. These agents were not available during the initial period of this study. Currently, these agents are used when there is some suspicion of a hepatic metastasis by sonography, CT, or non-contrast enhanced MR imaging, as well as in cases in which patients are eligible for hepatic resection.
In conclusion, this study demonstrated that preoperative unenhanced MR imaging has a high negative predictive value with regard to the detection of hepatic metastasis in patients who have undergone curative surgery for rectal cancer. The false-negative result was comparable to the results reported for IOUS. Therefore, the routine use of IOUS may not be warranted for those patients in whom hepatic metastasis was not suspected, based on the preoperative MR images.
Figures and Tables
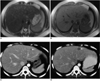 | Fig. 1A 43 year-old-woman with rectal cancer.
A, B. Preoperative MR images (T2-weighted single shot fast spin-echo image [17020/100] (A) and T1-weighted GRE image [230/4.0, 90° flip angle] (B)) show no evidence of metastasis in the liver.
C. Low attenuating subcentimetric lesion (white arrow) can be seen in the lateral segment on the follow-up CT scan, obtained three months postoperatively.
D. Growth of the lesion on the lateral segment (white arrow) can be seen on the CT scan at eight months postoperatively, which represents a metastasis.
|
Table 1
Histologic Stages of the Selected Patients Group
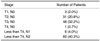
Note.-Histologic stages are as follows: T1, limited to mucosa and submucosa; T2, extension into but not through the muscularis propria; T3, invasion of perirectal fat; T4, invasion of adjacent structures; N0, no involved lymph nodes; N1, < four regional nodes positive for tumor; N2, > four regional nodes positive for tumor.
References
1. Welch JP, Donaldson GA. Detection and treatment of recurrent cancer of the colon and rectum. Am J Surg. 1978. 135:505–511.
2. Finlay IG, McArdle CS. Occult hepatic metastases in colorectal carcinoma. Br J Surg. 1986. 73:732–735.
3. Finlay IG, McArdle CS. The identification of patients at high risk following curative resection for colorectal carcinoma. Br J Surg. 1982. 69:583–584.
4. Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995. 19:59–71.
5. Sugarbaker PH. Surgical decision making for large bowel cancer metastatic to the liver. Radiology. 1990. 174:621–626.
6. Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997. 15:938–946.
7. Stewart PJ, Chu JM, Kos SC, Chapuis PH, Bokey EL. Intraoperative ultrasound for the detection of hepatic metastases from colorectal cancer. Aust N Z J Surg. 1993. 63:530–534.
8. Machi J, Isomoto H, Kurohiji T, et al. Accuracy of intraoperative ultrasonography in diagnosing liver metastasis from colorectal cancer: evaluation with postoperative follow-up results. World J Surg. 1991. 15:551–556. discussion 557.
9. Leen E, Angerson WJ, O'Gorman P, Cooke TG, McArdle CS. Intraoperative ultrasound in colorectal cancer patients undergoing apparently curative surgery: correlation with two year follow-up. Clin Radiol. 1996. 51:157–159.
10. Thaler W, Watzka S, Martin F, et al. Preoperative staging of rectal cancer by endoluminal ultrasound vs. magnetic resonance imaging. Preliminary results of a prospective, comparative study. Dis Colon Rectum. 1994. 37:1189–1193.
11. Satoh N, Ihara M, Sarashina H, et al. Studies of diagnosis of rectal cancer using MRI, CT and intrarectal ultrasonography. Rinsho Hoshasen. 1989. 34:573–581.
12. Kim NK, Kim MJ, Yun SH, Sohn SK, Min JS. Comparative study of transrectal ultrasonography, pelvic computerized tomography, and magnetic resonance imaging in preoperative staging of rectal cancer. Dis Colon Rectum. 1999. 42:770–775.
13. Plainfosse MC, Merran S. Work in progress: intraoperative abdominal ultrasound. Radiology. 1983. 147:829–832.
14. Gozzetti G, Mazziotti A, Bolondi L, et al. Intraoperative ultrasonography in surgery for liver tumors. Surgery. 1986. 99:523–530.
15. Castaing D, Emond J, Kunstlinger F, Bismuth H. Utility of operative ultrasound in the surgical management of liver tumors. Ann Surg. 1986. 204:600–605.
16. Machi J, Isomoto H, Yamashita Y, Kurohiji T, Shirouzu K, Kakegawa T. Intraoperative ultrasonography in screening for liver metastases from colorectal cancer: comparative accuracy with traditional procedures. Surgery. 1987. 101:678–684.
17. Boldrini G, de Gaetano AM, Giovannini I, Castagneto M, Colagrande C, Castiglioni G. The systematic use of operative ultrasound for detection of liver metastases during colorectal surgery. World J Surg. 1987. 11:622–627.
18. Conlon R, Jacobs M, Dasgupta D, Lodge JP. The value of intraoperative ultrasound during hepatic resection compared with improved preoperative magnetic resonance imaging. Eur J Ultrasound. 2003. 16:211–216.
19. Stone MD, Kane R, Bothe A Jr, Jessup JM, Cady B, Steele GD Jr. Intraoperative ultrasound imaging of the liver at the time of colorectal cancer resection. Arch Surg. 1994. 129:431–435. discussion 435-436.
20. Bergamaschi R, Arnaud JP. Routine compared with nonscheduled follow-up of patients with "curative" surgery for colorectal cancer. Ann Surg Oncol. 1996. 3:464–469.
21. Steele G Jr. Follow-up plans after treatment of primary colon and rectum cancer. World J Surg. 1991. 15:583–588.
22. Hamm B, Thoeni RF, Gould RG, et al. Focal liver lesions: characterization with nonenhanced and dynamic contrast material-enhanced MR imaging. Radiology. 1994. 190:417–423.
23. Mahfouz AE, Hamm B, Wolf KJ. Peripheral washout: a sign of malignancy on dynamic gadolinium-enhanced MR images of focal liver lesions. Radiology. 1994. 190:49–52.
24. Mathieu D, Rahmouni A, Anglade MC, et al. Focal nodular hyperplasia of the liver: assessment with contrast-enhanced TurboFLASH MR imaging. Radiology. 1991. 180:25–30.
25. Lee JM, Kim IH, Kwak HS, Youk JH, Han YM, Kim CS. Detection of small hypervascular hepatocellular carcinomas in cirrhotic patients: comparison of superparamagnetic iron oxide-enhanced MR imaging with dual-phase spiral CT. Korean J Radiol. 2003. 4:1–8.
26. Pena CS, Saini S, Baron RL, et al. Detection of malignant primary hepatic neoplasms with gadobenate dimeglumine (Gd-BOPTA) enhanced T1-weighted hepatocyte phase MR imaging: results of off-site blinded review in a phase-II multicenter trial. Korean J Radiol. 2001. 2:210–215.
27. Hamm B, Mahfouz AE, Taupitz M, et al. Liver metastases: improved detection with dynamic gadolinium-enhanced MR imaging? Radiology. 1997. 202:677–682.
28. Peterson MS, Baron RL, Murakami T. Hepatic malignancies: usefulness of acquisition of multiple arterial and portal venous phase images at dynamic gadolinium-enhanced MR imaging. Radiology. 1996. 201:337–345.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download