Abstract
Objective
Materials and Methods
Results
Conclusion
Figures and Tables
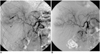 | Fig. 1A 48-year-old man presenting with an abruptly increased amount of fresh blood draining from a Jackson-Pratt drain. Two weeks previously, he underwent stent placement because of portal vein stenosis.
A. Common hepatic arteriogram shows a pseudoaneurysm (arrow) supplied by the segment V hepatic artery.
B. The supplying hepatic artery was successfully embolized with microcoils. After embolization, the patient clinically improved.
|
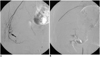 | Fig. 2A 47-year-old woman with arterial bleeding through a Jackson-Pratt drain.
A. Right 10th intercostal arteriogram shows extravasation of contrast media (arrow).
B. Right 10th intercostal artery was embolized with gelfoam particles and microcoils. After embolization, the stain disappeared. The patient clinically improved.
|
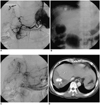 | Fig. 3A 47-year-old man presenting with arterial bleeding through a Jackson-Pratt drain.
A. Common hepatic arteriogram shows extravasation of contrast media (arrow).
B. A branch of the supplying hepatic artery was embolized with a straight microcoil.
C. Post-embolization hepatic arteriogram shows residual stain fed from fine collateral vessels.
D. Immediately after embolization, CT shows collection of contrast media along the hepatic resection margin. Afterwards, the patient underwent additional surgery to control the residual bleeding.
|
Table 1

Note.-S = technical success, F = technical failure, TSR = technical success rate, HA = hepatic artery, IHA = intrahepatic artery, HAAS = hepatic artery anastomotic site, HRM = hepatic resection margin, HJS = hepaticojejunostomy site, ICA = intercostal artery, IPA = inferior phrenic artery, SCA = thoracic branch of subclavian artery, SMAJ = jejunal branch of superior mesenteric artery, PDA = pancreaticoduodenal arcade, EIA = branch of external iliac artery
Table 2

Table 3
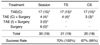
TAE = transcatheter arterial embolization, TS = technical success, CS = clinical success, TAE (C) = complete embolization, TAE (I) = incomplete embolization
*Parentheses means the changed values when three bleeding foci (hepaticojejunostomy site, hepatic arterial anastomotic site, and hepatic resection margin) were excluded.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download