Abstract
Isolated spontaneous dissection of the superior mesenteric artery (SMA) is a rare cause of acute mesenteric ischemia. Two patients were successfully treated by percutaneous stent placement within the main trunk of the SMA. Emphasis is placed on the feasibility of nonsurgical management with percutaneous stent placement of isolated spontaneous dissection of the SMA.
The spontaneous dissection of the main trunk of the superior mesenteric artery (SMA), when not associated with aortic dissection, is a rare cause of acute mesenteric ischemia. To our knowledge, less than fifty cases have been reported in literature. Most of these cases were managed surgically, or the patients died before surgery (1-2). In this report, two patients with acute mesenteric ischemia due to isolated spontaneous dissection of the SMA were successfully managed by percutaneous stent placement.
A 48-year-old woman suddenly experienced a diffuse rending abdominal pain, and so she visited the hospital. Her blood pressure was 190/130 mmHg and an antihypertensive drug was prescribed. Emergency contrast-enhanced abdominal CT revealed the presence of mural thrombus within the main trunk of the SMA without any evidence of abdominal aortic aneurysm or dissection (Fig. 1A). There was no finding of bowel ischemia such as bowel wall thickening or abnormal contrast enhancement. She was referred to our hospital 6 hours after her symptom's onset. Her medical history included hypertension, which had been controlled with a single drug for 4 years. The physical examination revealed a temperature of 36.8℃, blood pressure 110/70 mmHg, and the pulse was 68 beats per minute and regular. The abdomen showed diffuse mild tenderness. Routine lab findings were normal. An electrocardiogram, chest radiograph, and abdominal plain films were also normal. Under the impression of thromboembolic occlusion of the SMA, we tried catheter-directed thrombolytic therapy. The superior mesenteric arteriogram demonstrated the complete occlusion of the ileocolic and right colic branches of the SMA (Fig. 1B). An inferior mesenteric arteriogram showed collateral flow through a marginal artery to the occluded branches. A 5-F 11-cm multisidehole infusion catheter (COOK, Bloomington, IN, U.S.A.) was placed within the main trunk of the SMA and then urokinase was infused at a rate of 100,000 IU per hour. After 14-hours of infusion of urokinase, she still suffered from the symptoms, and the follow-up arteriogram revealed an isolated dissection of the SMA (Fig. 1C). We considered percutaneous stent placement instead of surgical bypass graft. Over the guidewire (Terumo, Tokyo, Japan) via a right femoral artery approach, an 8×70-mm self-expandable Wallstent (Boston Scientific, Watertown, MA, U.S.A.) was placed in the true lumen so that the proximal end was dipped into the aortic lumen (Fig. 1D). We used a relatively long stent because of the stability of this stent within the SMA. The control angiogram showed a patent, true lumen with good blood flow in all the branches of the SMA. The patient complained of postprandial abdominal pain for a week, however, the symptom was gradually subsided and the patient was discharged two weeks after the stent placement. Two months later, CT angiograms showed good blood flow through the fully expanded stent and all the branches of the SMA. However, the stent was shortened and the proximal segment of the SMA was narrowed by 50-75% (Fig. 1E), but the patient didn't complain of any symptoms for 6 months after the stent placement.
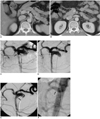
A 54-year-old healthy man experienced severe epigastric pain just after drinking a cup of cold water, and so he visited to our emergency room. His physical examination and routine lab values were negative. An electrocardiogram, chest radiograph and abdominal plain films were also normal. Routine abdominal US revealed only a mild fatty liver. Abdominal CT showed the common trunk of celiac and superior mesenteric arteries without evidence of bowel necrosis. An intimal flap was noted 1.5 cm distal to the orifice of the common trunk with a distally thrombosed and proximally patent false lumen (Figs. 2A, B). A celiacomesenteric arteriogram demonstrated a severely narrowed true lumen and an aneurysmally dilated false lumen (Fig. 2C). An 8×60-mm self-expandable Wallstent was placed in the true lumen to cover the dissection so that the proximal end of the stent was at the orifice of the SMA. The control angiogram showed a patent true lumen with good blood flow in all the branches of the SMA (Fig. 2D). Afterwards, his symptom was disappeared and he was discharged a week after the stent placement. Four months later, his celiacomeseteric arteriogram showed good blood flow through the stent, however, the aneurysmally dilated false lumen had recurred (Fig. 2E). To prevent the progression of the arterial dissection, another 10×50-mm self-expandable stent (S.M.A.R.T., Cordis, Miami, FL, U.S.A.) was placed within the Wallstent to cover the opening of the intimal flap (Fig. 2F). Three days later, a CT angiogram showed good patency through the fully expanded stents. The extent of the false lumen was significantly decreased and the tip of the latter stent was at the orifice of the celiacomesenteric trunk. The patient has had no complications for another two months.
The superior mesenteric artery is the second most frequent peripheral artery after the internal carotid artery to be affected by spontaneous dissection (2, 3). In the medical literature, the patients were predominantly male and over forty years of age (1, 2, 4). The reported risk factors of this disease were hypertension, cystic medial necrosis, abdominal aortic aneurysm and so on; however, most of the patients were generally healthy without underlying disease (5, 6).
When diagnosing SMA dissection, a contrast-enhanced CT scan can reveal the presence of an intimal flap and mural thrombus within the SMA lumen (3). However, because the intimal flap cannot be always noticed, the mural thrombus may be only the clue of the SMA dissection. In our former case, we misdiagnose the dissection as the thromboembolic occlusion of the SMA and then we preformed an unnecessary pharmacologic thrombolysis. Therefore, the arterial dissection must be included as one of differential diagnosis in cases of mural thrombosis of the SMA.
The mainstay of treatment of the spontaneous dissection of the SMA has been surgery (1, 2). Surgical techniques have included graft interposition, reimplantation of SMA on the aorta, intimectomy, right gastroepiploic artery bypass, and simple arteriotomy with thrombectomy (1). However, surgery was generally accompanied with a variety of complications. Recently, successful conservative management has been reported, although long-term results have not been obtained (2, 4).
Several authors have shown promising results for the stenting of chronic lesions of the visceral arteries (7, 8). Leung et al. (2) have reported the first successful case of isolated spontaneous dissection of the SMA treated by percutaneous stent placement. The patient didn't experiencea recurrence of abdominal angina during a 6-month follow-up period. However, in acute mesenteric ischemia due to arterial dissection, the clinical outcome and complications after the stent placement cannot be anticipated yet because of an insufficient number of patients.
In our cases, the Wallstent showed one important disadvantage, and that is proximal shortening. For our female patient, the uncovered proximal SMA became narrowed two months later. The mechanism is not certain, however, suspected mechanisms may be the proximal shortening of the non-fixed wide proximal SMA segment and an irritation of the vessel wall by the free end of the stent. For our male patient, the opening of the false lumen wasn't covered by the stent due to proximal shortening, so that the aneurysmally dilated false lumen compressed the stent. Solis et al. reported that the dissection usually begins 1.5-3 cm from the orifice of the SMA, thereby sparing the origin of the artery. This segment of the SMA corresponds with the portion running behind the pancreas, and being a fixed site, it may bear the consequences of a straining force (1). Therefore, this proximal SMA segment must be securely covered by the stent. In our latter patient, an additional stent with less shortening and a greater ability to conform was needed to cover the intimal flap. Consequently, the stent for the SMA dissection needs several important characteristics; minimal shortening, good conformability and flexibility, and it must not change location due to the continuous movement of the mesentery. As in aortic dissection, most of cases of segmental dissection without side branch involvement or distal reentry may benefit from implanting a covered stent graft over the entry site of the dissection, thus causing thrombosis and obliteration of the false lumen (9).
Multidetector row CT facilitates shorter acquisition times, retrospective creation of thinner sections from the raw data, and improved three-dimensional rendering with diminished helical artifacts (10). In our cases, the three-dimensional CT angiography provided sufficient information for the assessment of the spontaneous dissection of the SMA. These techniques were also useful for our follow-up examination after the stenting. Concerning conventional arteriography, catheterization into the dissected peripheral artery at an early stage may worsen the arterial tear. In our opinion, the conventional diagnostic angiography will soon be replaced with CT angiography.
In conclusion, percutaneous stent placement is a safe and feasible therapeutic alternative for the treatment of isolated spontaneous dissection of the SMA. However, studies with a larger group of patients and long-term follow-up will be needed to validate this procedure.
Figures and Tables
Fig. 1
A 48-year-old woman with sudden abdominal pain.
A. The contrast-enhanced CT scan showed mural thrombus (arrow) within the main trunk of the superior mesenteric artery.
B. Superior mesenteric arteriogram demonstrated the complete occlusion of ileocolic and right colic branches of the SMA.
C. After continuous infusion of urokinase into the superior mesenteric artery, arteriograms revealed the intimal flap (arrow).
D. An 8×70-mm self-expandable Wallstent was placed in the true lumen so that the proximal stent was dipped into the aortic lumen (arrow).
E. Two months after stent placement, the maximum intensity projection image demonstrated the proximal shortening of the stent and the stenosis of the SMA orifice (arrow).
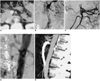
Fig. 2
54-year-old man with severe epigastric pain after drinking cold water.
A, B. The contrast-enhanced CT scan showed the intimal flap (long arrow) and the mural thrombus (short arrow).
C. The celiacomesenteric arteriogram demonstrated a severely narrowed true lumen (long arrow) and aneurysmally dilated false lumen (short arrow).
D. After the stent placement, the proximal end was located at the orifice of the SMA (arrow). The control angiogram showed a patent true lumen with good blood flow in all the branches of the SMA, and an almost vanished false lumen.
E. At 4 months after the stent placement, the arteriogram showed the recurrence of aneurysmally dilated false lumen (arrow).
F. Another 10-50-mm self-expandable stent (arrow) was placed within the Wallstent covering the opening of intimal flap. The aneurysmally dilated false lumen was significantly decreased.
References
1. Sparks SR, Vasquez JC, Bergan JJ, Owens EL. Failure of nonoperative management of isolated superior mesenteric artery dissection. Ann Vasc Surg. 2000. 14:105–109.
2. Leung DA, Schneider E, Kubik-Huch R, Marincek B, Pfammatter T. Acute mesenteric ischemia caused by spontaneous isolated dissection of the superior mesenteric artery: treatment by percutaneous stent placement. Eur Radiol. 2000. 10:1916–1919.
3. Kodaira M, Fukaya T. Gastrointestinal: isolated and spontaneous dissection of the superior mesenteric artery. J Gastroenterol Hepatol. 2001. 16:933.
4. Yasuhara H, Shigematsu H, Muto T. Self-limited spontaneous dissection of the main trunk of the superior mesenteric artery. J Vasc Surg. 1998. 27:776–779.
5. Hyodoh H, Hyodoh K, Takahashi K, Yamagata M, Kanazawa K. Three-dimensional CT imaging of an isolated dissecting aneurysm of the superior mesenteric artery. Abdom Imaging. 1996. 21:515–516.
6. Common AA, Pressacco J. Chronic dissection of the superior mesenteric artery: case report. Can Assoc Radiol J. 1999. 50:23–25.
7. Nyman U, Ivancev K, Lindh M. Endovascular treatment of chronic mesenteric ischemia: report of five cases. Cardiovasc Intervent Radiol. 1998. 21:305–313.
8. Maleux G, Wilms G, Stockx L, Vancleemput J, Baert L. Percutaneous recanalization and stent placement in chronic proximal superior mesenteric artery occlusion. Eur Radiol. 1997. 7:1228–1230.
9. McGraw JK, Patzik SB, Gale SS, Dodd JT, Boorstein JM. Autogenous vein-covered stent for the endovascular management of a superior mesenteric artery pseudoaneurysm. J Vasc Interv Radiol. 1998. 9:779–782.
10. Furukawa H, Moriyama N. Spontaneous dissection of the superior mesenteric artery diagnosed on multidetector helical CT. J Comput Assist Tomogr. 2002. 26:143–144.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download