Abstract
Objective
To determine whether saline-enhanced dual probe bipolar radiofrequency ablation (RFA) using perfused-cooled electrodes shows better in-vitro efficiency than monopolar or single probe bipolar RFA in creating larger coagulation necrosis.
Materials and Methods
RF was applied to excised bovine livers in both bipolar and monopolar modes using a 200W generator (CC-3; Radionics) and the perfused-cooled electrodes for 10 mins. After placing single or double perfused-cooled electrodes in the explanted liver, 30 ablation zones were created at three different regimens: group A; saline-enhanced monopolar RFA, group B; saline-enhanced single probe bipolar RFA, and group C; saline-enhanced dual probe bipolar RFA. During RFA, we measured the tissue temperature at 15mm from the electrode. The dimensions of the ablation zones and changes in the impedance currents and liver temperature during RFA were then compared between the groups.
Results
The mean current values were higher for monopolar mode (group A) than for the bipolar modes (groups B and C): 1550±25 mA in group A, 764±189 mA in group B and 819±98 mA in group C (p < 0.05). The volume of RF-induced coagulation necrosis was greater in group C than in the other groups: 27.6±2.9 cm3 in group A, 23.7±3.8 cm3 in group B, and 34.2±5.1 cm3 in group C (p < 0.05). However, there was no significant difference between the short-axis diameter of the coagulation necrosis in the three groups: 3.1±0.8 cm, 2.9±1.2 cm and 4.0±1.3 cm in groups A, B and C, respectively (p > 0.05). The temperature at 15 mm from the electrode was higher in group C than in the other groups: 70±18℃ in group A, 59±23℃ in group B and 96±16℃ in group C (p < 0.05).
The major limitation of the existing RF technology is its incapability to produce a coagulation area that is large enough to include focal liver malignancies and a reasonable safety margin (5-10 mm) of seemingly normal tissue (1, 2). To overcome this limitation of monopolar RFA, various approaches have been tried, including saline-enhanced RFA (3, 4), multiple probe RFA applied in the form of either simultaneous (5) or alternative RFA (6), and bipolar RFA (7-10).
Recently, there have been several studies which demonstrated that saline-enhanced bipolar RFA showed superior performance in creating coagulation necrosis (7, 11, 12). There are two different approaches which can be used for saline-enhanced bipolar RFA, depending on whether a single bipolar electrode (7) or dual electrodes are used (11, 12). Although each technique has its own advantages, to the best of our knowledge no studies have been published comparing the efficacy of saline-enhanced monopolar RFA, saline-enhanced single probe bipolar RFA and dual probe bipolar RFA. In this context, we compared the tissue heating ability and dimensions of coagulation necrosis for these different current configurations.
Based on the results of previous studies of monopolar RFA using a cooled wet electrode (13, 14) and bipolar RFA using a single bipolar electrode (7), we developed two types of perfused-cooled electrodes, by modifying a 17-gauge internally cooled electrode (Radionics, Burlington, Mass., U.S.A.), in order to allow the use of both saline interstitial infusion and intra-electrode cooling. In this context, we conducted a comparative study to examine whether saline-enhanced bipolar RFA, using either single or dual perfused-cooled electrodes, creates a larger dimension of coagulation necrosis than saline-enhanced monopolar RFA.
Considering the results of a previous study performed by Ni et al. (13), we developed three types of perfused-cooled electrodes which permit both intra-electrode cooling perfusion and interstitial saline infusion to be performed (Fig. 1). For monopolar RFA, we modified a 17-gauge cooled-tip electrode with a 4-cm active tip (Radionics), by completely covering its shaft, except for the active tip portion, with a 15-gauge outer sheath, which was electrically insulated by coating it with polyteflon. The space between the 15-gauge sheath and the cooled tip electrode permitted saline infusion along the electrode. A side hole located in the proximal part of the outer sheath was used for saline infusion.
For the dual probe bipolar RFA, a 17-gauge cooled-tip electrode with a 2-cm active tip was used with some modification. In dual probe bipolar mode, one of two probes was used as the dispersive electrode. For singe probe bipolar RFA, the outer sheath acted as the dispersive electrode. Both the inner cooled-tip electrode and the outer sheath had a 2 cm-long exposed portion. The remaining portion of the shaft was electrically insulated by coating it with polyteflon (Fig. 1).
Because of the consistent ablation results which have been reported for ex vivo RFA experiments (14, 15), we chose excised bovine liver, purchased from a local butcher, as the RFA target. RFA was performed in ten freshly excised bovine livers weighing, on average, 7 Kg. The livers were cut into several 10×10×7-cm3 blocks, which were immersed into a 50×20-cm saline (36.5℃)-filled bath. Thereafter, a 15-gauge perfused-cooled bipolar electrode or a monopolar electrode with a 4 cm-active tip was placed in the liver. The tips of the electrodes were introduced at least 5 cm into the target tissue. In the case of the dual probe bipolar RFA, two perfused-cooled electrodes were placed at a 3-cm inter-electrode spacing, according to our preliminary study results (unpublished data). To continuously measure the local tissue temperature during the procedure, a thermocouple was inserted at a position 15 mm from the electrode. The tissue impedance was monitored using circuitry incorporated into the generator.
A 480 kHz RF generator with a maximum power output of 200 W (CC-3; Radionics) was used in all experiments, with the simultaneous registration of impedance during energy delivery. Based on a previous study, the pulsed RF application technique was used (16). To compare the efficacy of bipolar RFA using the perfused-cooled bipolar electrode with that of monopolar RFA with saline infusion for the creation of coagulation necrosis, 10 ablation zones were created with each of the following conditions: group A-hypertonic saline-enhanced monopolar RFA; group B-hypertonic saline-enhanced single probe bipolar RFA; group C-hypertonic saline-enhanced dual probe bipolar RFA.
In the case of monopolar RFA, a 15-gauge perfused cooled electrode with a tip exposure of 4 cm was used, with using hypertonic saline infusion. The distance between the electrodes and the dispersive metallic pad was adjusted to create an initial tissue impedance of 80Ω(the mean distance between the electrode and pad was 35 cm). The RF power was increased manually to 150 watts, and RF energy was applied for ten minutes in the monopolar mode. In this mode, current flows from one electrode to a dispersive metallic pad. Hypertonic saline (6%) was infused at a rate of 1 mL/min through a perfused-cooled electrode using an infusion pump (Pilotec IS; Fresenius Medical Care, Alzenau, Germany). A peristaltic pump (Watson-Marlow, Medford, Mass., U.S.A.) was used to infuse 0℃ saline solution into the lumen of the electrodes at a rate sufficient to maintain a tip temperature of 20-25℃.
In the case of the single probe bipolar RFA, the electrode was placed in the liver without a dispersive pad. In this mode, current flows from the distal tip of the electrode to an uninsulated portion of outer metallic sheath. Using an infusion pump (Pilotec IS) 6% hypertonic saline was infused at a rate of 1 mL/m through the perfused-cooled electrode.
In the case of the dual probe bipolar RFA, two open-perfused electrodes were placed in the liver tissue 3 cm apart and without a dispersive pad, and were attached to the generator. In this mode, current flows from one electrode to the other. Based on our unpublished data, the distance between the two electrodes placed in the liver was 3 cm and they were inserted through an acryl plate containing multiple holes at 5 mm intervals. Using two infusion pumps (Pilotec IS), 6% hypertonic saline was infused at a rate of 0.5 mL/m through the perfused-cooled electrode.
The applied current, power output and impedance were continuously monitored during the RF ablation, and were recorded automatically using a computer program (Real Time Graphics Software V 2.0; Radionics). The technical parameters of the RFA procedure, including the impedance and wattage changes, tissue temperature at the midpoint were compared for the four techniques.
Liver blocks containing RF ablation lesions, were dissected along the longitudinal plane (L-plane) passing through the axes of both probes. Since the white central area of the RF induced ablation zone has been previously shown to correspond to the zone of coagulation necrosis (17), two observers measured the vertical diameter (Dv) along the probes, and the transverse diameter (Dt) perpendicular to the Dv in the L-plane. After measuring these two diameters of the ablation zone in the L-plane, the dissected liver specimens were cut transversely, perpendicular to the L-plane (T-plane) through the midpoint of the ablation sphere. Then, the short-axis diameter of the ablation zone (Ds) was measured in the T-plane. The volumes of the ablation zones were evaluated by approximating the lesion to a sphere using the formula: π (Dv×Dt×Ds)/ 6. The shape of the RF-induced ablation zone was characterized using the Dt/Dv ratio.
The dimensions of the thermal ablation area and the technical parameters of the four groups were averaged for each group and compared using one-way analysis of variance (ANOVA) with the Dunnet test (p = 0.05, two-tailed test). The values were expressed as means±S.D. To compare the temperature at the midpoint between the two electrode tips, the repeated measure of ANOVA test was performed. For all statistical analyses, a p value of less than 0.05 was considered significant. Statistics were performed using the Instat program (GraphPad Software, Inc., San Diego, Cal., U.S.A.).
During the application of RF energy in group A, the tissue impedance was gradually decreased to 50Ω by means of hypertonic saline infusion. In the bipolar modes, using either a single probe or double probes, the tissue impedance was increased to more than 150Ω, 4-6 minutes after starting the RFA, and pulsed RF application was used. The mean accumulated energy outputs for each group were 1550±25 mA in group A, 764±189 mA in group B and 819±98 mA in group C. The differences in the mean current between group A and the other groups were statistically significant (p < 0.05).
Graphs showing the mean temperature at the midpoint between the two electrode tips are shown in Figure 2. The mean final midpoint temperature values were 70±18℃ in group A, 59±23℃ in group B, and 96±16℃ in group C (p < 0.05).
After RFA, a well-defined area with a central white discoloration was observed in the ablated zone sections. The mean Dv values of the RF induced central white zone, measured in the gross specimens of the three groups, were as follows: 5.0±0.7 cm in group A, 5.2±1.2 cm in group B and 3.8±0.8 cm in group C. The differences in the Dv values between groups A and C, and between groups B and C were statistically significant (p < 0.05) (Table 1). The mean Dt values were 3.4±0.9 cm in group A, 3.0±1.1 cm in group B and 4.3±1.3 cm in group C (Fig. 3). There was a significant difference in the Dt value between groups B and C (p < 0.05). The mean Ds values of the ablated spheres were 3.1±0.8 cm, 2.9±1.2 cm and 4.0±1.3 cm in groups A, B and C, respectively. The differences between the groups were not significant (p > 0.05). However, saline-enhanced dual probe bipolar RFA (group C) created ablation zones with a significantly larger volume than either monopolar-(group A) or single probe bipolar RFA (group B): 27.6±2.9 cm3 in group A, 23.7±3.8 cm3 in group B and 34.2±5.1 cm3 in group C. The differences between the volume of group C and those of the other groups were statistically significant (p < 0.05).
The Dt/Dv ratio was 0.68±0.1 in group A, 0.58±0.2 in group B and 1.13±0.2 cm in group C, and the differences between groups A and C, and between groups B and C were significant (p < 0.05). This means that the dual probe bipolar systems using both intra-electrode cooling and the interstitial saline infusion function of the perfused-cooled electrodes (group C) tended to produce a more sphere-shaped coagulation than that which was obtained in the other groups.
With the currently available RF techniques, it is not always possible to achieve an area of destruction which extends 0.5 to 1 cm beyond the targeted tumor, in order to provide the equivalent of a surgical margin (1, 2). Since performing overlapping ablations may be both difficult and cumbersome under ultrasound guidance, there have been many attempt to develop new types of electrodes that can induce a larger coagulation necrosis by means of a single RF application, including perfused electrodes (18), expandable-wet electrodes (19), cooled-wet electrodes (13, 14) and saline-enhanced bipolar single electrodes (7). Among these approaches, the saline-enhanced bipolar electrode seems to be the most promising, because many previous studies have documented the improved performance obtained by using saline infusion during RFA and by using bipolar RFA compared to standard monopolar RFA.
In this study, we developed three different types of perfused-cooled electrodes, which are variously designed for saline-enhanced monopolar and bipolar RFA. At the time we developed the perfused-cooled electrode for bipolar RFA, we referenced several previous studies regarding the electrode configuration (12-16). Based on the results of these previous studies, we supposed that the use of bipolar mode RFA combined with the newly developed perfused-cooled electrode, which permits simultaneous intra-electrode cooling and interstitial saline infusion, would improve the efficacy of RF mediated coagulation necrosis.
Our study demonstrated that saline-enhanced dual probe bipolar RFA showed a larger Dt/Dv ratio than the other techniques using a single electrode. Therefore, in bipolar mode, the shape of the ablation zones was more spherical with dual probe RFA than with single probe RFA. This improvement was attributed to the difference in length of the active portion of the single monopolar electrode compared with that of the single bipolar electrode. Based on a previous study by Goldberg et al. (20), the electrode with the longer active tip created oval or spindle-shaped coagulation necrosis. Given that the transverse diameter of the RF-induced coagulation necrosis is more critical to achieving complete necrosis of the treated tumor, bipolar RFA using dual probes appears to be superior to single monopolar or single bipolar RFA.
In addition, bipolar RFA using dual probes created a coagulation with a larger volume than both saline-enhanced monopolar RFA and bipolar RFA using a single probe (p < 0.05). This can be attributed to two factors. First, considering that the mean current delivered to the tissue during dual probe bipolar RFA (819±98 mA) was much smaller than that during monopolar RFA (1550±25 mA), the larger dimension of coagulation necrosis achieved by dual bipolar RFA can be explained by the greater energy efficacy of dual probe bipolar RFA compared with that of monopolar RFA at the same Watt delivery (11, 21). While in dual probe bipolar mode, the current should flow from the active electrode to the dispersive electrode through the liver tissue between the two electrodes, in single probe bipolar mode, the current flows though the infused hypertonic saline, which accumulates in the adjacent space along the electrode between the two uninsulated portion of the electrode. Therefore, because the electrical resistance of the hypertonic saline is much smaller than that of the liver tissue, current flows along the hypertonic saline, and this results in less conversion of electrical energy into thermal energy in the liver tissue. Second, in monopolar mode or single probe bipolar mode, heat is diverted from the ablation site in all directions, while in dual probe bipolar mode, one electrode is thermally shielded by the opposing second electrode, which also actively heats the tissue in its proximity; therefore, heat is trapped between the two electrodes and a higher temperature is thus achieved, and less cooling occurs in the direction of the collateral electrode than is the case with monopolar ablation (22).
There are several modes that can be employed when using dual electrodes for RF ablation. The first method involves the application of RF energy continuously to one electrode for a certain time period, and then to the other (sequential mode) in a monopolar fashion, which prolongs the procedure time. The second method involves the synchronous application of energy to both electrodes in a monopolar fashion (simultaneous monopolar mode). The third option involves the application of RF energy alternatively, by delivering the RF current alternately to each electrode (alternative mode) (6). The fourth option is to apply RF energy to one electrode only and to use the other electrode as the return electrode (bipolar mode). Simultaneous monopolar mode, alternative-monopolar mode and bipolar mode have been shown to create larger lesions than monopolar ablation when using a single-needle electrode (6, 11, 23). Haemmerich et al. (22) demonstrated that the alternative monopolar and bipolar modes showed better efficacy than simultaneous monopolar mode. Considering that in simultaneous monopolar mode, there is a relatively low field gradient between the two electrodes, which may result in reduced power delivery to the area between the electrodes, we believe that the bipolar mode has the potential to provide better performance than the simultaneous monopolar mode. Further comparative studies between the saline-enhanced alternative monopolar- and saline-enhanced bipolar modes are warranted in order for the size of the coagulation necrosis to be increased.
Bipolar RFA using dual probes may also have some disadvantages as compared to monopolar - or bipolar RFA using a single probe. First, in bipolar RFA using dual probes, the probes must be parallel to each other, with the tumor situated between them. However, in the clinical situation, the insertion of two parallel probes can sometimes be difficult to achieve. In addition, using two probes increases the expense of the RFA procedure, and also increases the risk of complications related to the insertion of the probe, such as bleeding or traumatic injury to the treated organ. Secondly, in the bipolar mode, there is no way to independently control the amount of heat generated in the vicinity of the probes. When the degree of cooling at the two electrodes is different, for example, due to differences in perfusion, one probe may reach a higher temperature than the other, and this can lead to boiling and a rapid increase in impedance.
Our experimental study has certain limitations. Firstly, all of the ablations were performed in vitro. Living tumor tissue, has a cooling "sink" effect due to blood flow, thus rapid heat exchange can occur (24, 25). Therefore, the extent to which the results of this experimental study can be extrapolated to the real RFA situation in humans may be limited. In spite of this limitation, however, our in vitro experiments provide a reliable basis of comparison between the relative efficiencies of the different current delivery modes. Secondly, all of our tests were performed using 6% NaCl solution at a flow rate of 1 mL/min. We believe that further experimental studies are warranted, with the purpose of optimizing the concentration and the amount of hypertonic saline solution. Finally, the generator we used for bipolar RFA was originally invented for and optimized for monopolar RFA and, therefore, further modification of generator may be required.
In conclusion, saline-enhanced dual probe bipolar RFA produces significantly larger ablation zones than monopolar RFA. In addition, it generates larger ablation zones than bipolar RFA using a single electrode. This ability of dual probe bipolar RFA to produce larger coagulation necrosis could be valuable for clinical RF application in cases involving large liver tumors.
Figures and Tables
Fig. 1
Photograph of the electrode used for saline-enhanced monopolar mode (group A) with a 4-cm active tip (upper), the electrode used for dual probe bipolar mode (group C) with a 2-cm active tip (middle) and a single bipolar electrode used for single probe bipolar mode (group B) with two 2-cm uninsulated portions (bottom).

Fig. 2
A graph showing the changes in mean temperature at 15 mm from the electrode tips in each group during radiofrequency ablation: group A = hypertonic saline-enhanced monopolar RFA; group B = hypertonic saline-enhanced single probe bipolar RFA; group C = hypertonic saline-enhanced dual probe bipolar RFA.

Fig. 3
Comparison of radiofrequency-induced coagulation in the three groups. Note that the mean long-axis coagulation diameters were larger in group C than in the other groups
A. Photograph of specimen from group A (hypertonic saline-enhanced monopolar RFA)
B. Photograph of specimen from group B (hypertonic saline-enhanced single probe bipolar RFA)
C. Photograph of specimen from group C (hypertonic saline-enhanced dual probe bipolar RFA)
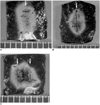
Table 1
Measured Values of RF-induced Coagulation Necrosis for the Three Different Modes

Note.-Dt: transverse-axis diameter, Dv: vertical-axis diameter, Ds: short-axis diameter, group A: Saline-enhanced monopolar RFA, group B: saline-enhanced single probe bipolar RFA, group C: saline-enhanced dual probe bipolar RFA.
*The difference between groups B and C was statistically significant, †the differences between groups A and C, and groups B and C were statistically significant, ‡the differences between group C and the other groups were statistically significant.
References
1. Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound. 2001. 13:129–147.
2. Dodd GD, Frank MS, Aribandi M, Chopra S, Chintapalli KN. Radiofrequency thermal ablation: computer analysis created by overlapping ablations. AJR Am J Roentgenol. 2002. 177:777–782.
3. Lee JM, Kim YK, Lee YH, Kim SW, Li CA, Kim CS. Percutaneous radiofrequency thermal ablation with hypertonic saline injection: in-vivo study in a rabbit liver model. Korean J Radiol. 2003. 4:27–34.
4. Munver R, Threatt CB, Delvecchio FC, Preminger GM, Polascik TJ. Hypertonic saline-augmented radiofrequency ablation of VX2 tumor implanted in rabbit kidney: a short-term survival pilot study. Urology. 2002. 60:170–175.
5. Goldberg SN, Solbiati L, Hahn PF, Cosman E, Conrad JE, Fogle R, et al. Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases. Radiology. 1998. 209:371–379.
6. Lee JM, Rhim H, Han JK, Youn BJ, Kim SH, Choi BI. Dual-probe radiofrequency ablation: an in vitro experimental study in bovine liver. Invest Radiol. 2004. 39:89–96.
7. Burdio F, Guemes A, Burdio JM, et al. Bipolar saline-enhanced electrode for radiofrequency ablation: results of experimental study of in vivo porcine liver. Radiology. 2003. 229:447–456.
8. McGahan JP, Gu WZ, Brock JM, Tesluk H, Jones CD. Hepatic ablation using bipolar radiofrequency electrocautery. Acad Radiol. 1996. 3:418–422.
9. Curley SA, Davidson BS, Fleming RY, et al. Laparoscopically guided bipolar radiofrequency ablation of areas of porcine liver. Surg Endosc. 1997. 11:729–733.
10. Haemmerich D, Staelin ST, Tungjitkusolmun S, Lee FT, Mahvi DM, Webster JG. Hepatic bipolar radiofrequency ablation between separated multiprong electrodes. IEEE Trans BioMed Eng. 2001. 48:1145–1152.
11. Lee JM, Han JK, Kim SH, et al. A comparative experimental study of the in-vitro efficiency of hypertonic saline-enhanced hepatic bipolar and monopolar radiofrequency ablation. Korean J Radiol. 2003. 4:163–169.
12. Burdio F, Guemes A, Burdio JM, et al. Hepatic lesion ablation with bipolar saline-enhanced radiofrequency in the audible spectrum. Acad Radiol. 1999. 6:680–686.
13. Ni Y, Miao Y, Mulier S, Yu J, Baert AL, Marchal G. A novel "cooled-wet" electrode for radiofrequency ablation. Eur Radiol. 2000. 10:852–854.
14. Miao Y, Ni Y, Yu J, Marchal G. A comparative study on validation of a novel cooled-wet electrode for radiofrequency liver ablation. Invest Radiol. 2000. 35:438–444.
15. Lee JM, Han JK, Kim SH, et al. Wet radio-frequency ablation using dual electrodes: comparative study of bipolar mode versus monopolar simultaneous and alternating modes in the bovine liver. EJR. 2004. (in press).
16. Goldberg SN, Stein MC, Gazelle GS, Sheiman RG, Kruskal JB, Clouse ME. Percutaneous radiofrequency tissue ablation: optimization of pulsed-radiofrequency technique to increase coagulation necrosis. J Vasc Interv Radiol. 1999. 10:907–916.
17. Lee JD, Lee JM, Kim SW, Kim CS, Mun WS. MR imaging-histopathologic correlation of radiofrequency thermal ablation lesion in a rabbit liver model: observation during acute and chronic stages. Korean J Radiol. 2001. 2:151–158.
18. Kettenbach J, Kostler W, Rucklinger E, et al. Percutaneous saline-enhanced radiofrequency ablation of unresectable hepatic tumors: initial experience in 26 patients. AJR Am J Roentgenol. 2003. 180:1537–1545.
19. Miao Y, Ni Y, Yu J, Zhang H, Baert A, Marchal G. An ex vivo study on radiofrequency tissue ablation: increased lesion size by using an "expandable-wet" electrode. Eur Radiol. 2001. 11:1841–1847.
20. Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques and diagnostic imaging guidance. AJR Am J Roentgenol. 2000. 174:323–331.
21. Goldberg SN, Ahmed M, Gazelle GS, et al. Radiofrequency thermal ablation with NaCl solution injection: effect of electrical conductivity on tissue heating, and coagulation-phantom and porcine liver study. Radiology. 2001. 219:157–165.
22. Haemmerich D, Tungjitkusolmun S, Staelin ST, Lee FT Jr, Mahvi DM, Webster JG. Finite-element analysis of hepatic multiple probe radio-frequency ablation. IEEE Trans Biomed Eng. 2002. 49:836–842.
23. Jang IS, Rhim H, Koh BH, et al. An experimental study of simultaneous ablation with dual probes in radiofrequency thermal ablation. J Korean Radiol Soc. 2003. 48:163–169. (in Korean).
24. Goldberg SN, Hahn PF, Halpern EF, Fogle RM, Gazelle GS. Radiofrequency tissue ablation: effect of pharmacologic modulation of blood flow on coagulation diameter. Radiology. 1998. 209:761–769.
25. Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998. 227:559–565.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download