This article has been retracted. See "Notice of Redundant Publication" in Volume 8 on page 262.
Abstract
Objective
The aim of this study was to determine if snorers have a narrower oropharyngeal airway area because of fat infiltration, and an elevated body mass index.
Materials and Methods
Ten control subjects and 19 patients that snored were evaluated. We obtained 2-mm-thick axial CT scan images every 0.6 seconds during expiration and inspiration at the same level of the oropharynx. We selected the largest and the smallest oropharyngeal airway areas and found the differences. From the slice that had the smallest oropharyngeal airway area, the thickness of the parapharyngeal and subcutaneous fat was measured. The measurements from the left and right side were added together and single values for parapharyngeal and subcutaneous fat tissue thickness were then found.
Results
The conventional measure of body mass index was significantly higher in the snorers (p < 0.05). The difference in the smallest oropharyngeal airway area between snorers and the controls was statistically significant (p < 0.01). The average difference between the largest and the smallest oropharyngeal area in the control group and the snorer group was statistically significant (p < 0.05). There was no significant difference in the largest oropharyngeal airway area, the total subcutaneous fat width and the total parapharyngeal fat width between snorers and control subjects (p > 0.05).
Snoring occurs in all age groups, in both genders, and it is heard, literally, all over the world. Snoring often puts a strain on family or social relationships and is a frequent source of embarrassment. Snoring is undoubtedly the most frequent complaint of patients with obstructive sleep apnea, which usually leads them to a sleep laboratory. This close link between snoring and sleep apnea has delayed our understanding of the possible independent adverse medical consequences of snoring, simply because snoring has been invariably discussed in the context of sleep apnea (1). The most common risk factors for snoring are male gender, obesity, ingestion of tranquillisers or muscle relaxants, smoking and alcohol consumption. Knowledge of these risk factors should prompt the physician to inquire about the presence of such factors (2, 3). Laboratory investigation is difficult issue, and no consensus on the causes of snoring have yet been reached. Two investigations that are usually contemplated and attempted in snoring patients are nocturnal polysomnography and airway assessment (1). Symptomatic snorers also complain of excessive sleepiness, tiredness and fatigue, or their partners report episodes of cessation of breathing. Symptomatic snorers have an increased likelihood of having sleep apnea. Pioneering investigations have elevated snoring from the level of social nuisance to a disease state (1). Snoring and sleep apnea may be a risk factor for vascular diseases, such as hypertension, cerebrovasculer disease and coronary artery disease (1, 4-6). It has been suggested that asthmatics who snore and have nocturnal asthma attacks may have unsuspected sleep apnea, which can trigger these attacks (1).
It has also been suggested that subjects with snoring or obstructive sleep apnea have a narrower pharyngeal airway than do normal persons because of fat infiltration, decrease pharyngeal muscle tone, or the weight of the soft tissue of the neck (7-11).
The purpose of this study was to validate the premise that snorers may have a smaller oropharyngeal airway in relation to increased fat infiltration and an elevated body mass index. We investigate our hypothesis by using spiral-computed tomography (CT), a digitising instrument and its measure tools.
Nineteen snorers (14 men and 5 woman) who were followed by the Otolaryngology Department and 10 control subjects (7 men and 3 woman) were selected as the study subjects (Table 1). The mean ages were 39 years (range 24 to 58) and 40 years (range 22 to 51), respectively. Patients with snoring were untreated at the time they were studied. Exclusion criteria were an age less than 18 years, being a radiation industry worker, a history of thyroid disease, and pregnancy. All subjects underwent a history and physical examination. The volunteer control subjects were selected from these with a regular sleeping partner that was able to confirm the control subjects did not habitually snore. A detailed medical and snoring history was taken. All patients underwent measurements of weight and height. Body mass index (BMI) was used as an index of obesity. It was calculated by dividing a subject's mass in kilograms by the square of a subject's height in meters. Three of the snorers and one of control subjects were obese (BMI was greater than 30). Eleven of snorers and four of controls were overweight (BMI was 25-29, 9).
Each subject had an awake spiral CT scan (Xpress/GX model TSX 002a Toshiba, Toshigi-Ken) of the upper airway. The subject's head was positioned with the soft tissue Frankfort plane (tragus of the ear to soft tissue orbitale) perpendicular to the floor. The subjects were closely observed to ensure that they remained awake throughout the procedure and did not swallow or talk during imaging. Intravenous contrast material was not administered. We performed a preview scan that yielded anterior-posterior and sagital projection images to locate the oropharyngeal anatomic level to be scanned. CT scans were obtained at the oropharyngeal anatomic level while the subjects were quietly breathing through the nose. Scanning encompassed four or five full respiratory cycles in all subjects and at least 24 scans were obtained at the same anatomic level. Scanning was performed to obtain 2-mm-thick axial CT slices every 0.6 seconds during expiration and inspiration at the same level of the oropharynx. All scans were obtained using the single slice technique. The table speed was 0. Window settings were standardized for all subjects with a window level of 50 HU and a window width of 250 HU.
We chose two slices on cine-CT images, one showing the largest airway area that matched the early expiration, and the other displaying the smallest airway area that matched the late expiration and beginning of the inspiration (12). We calculated the oropharyngeal areas on these two slices and found the difference of the areas.
The thickness of the parapharyngeal and subcutaneous fat was measured from the slice that had the smallest oropharyngeal airway area (Fig. 1). The measurements from the left and right sides were added together and single values for parapharyngeal and subcutaneous fat tissue thickness were found and these values were used for comparison. We drew a line, perpendicular to the middle of the long axis of the masseter muscle, and on this perpendicular line we measured the subcutaneous fat width. We measured the distance between the parallel lines, which was drawn tangent to medial and lateral contours of parapharyngeal fat pad.
We compared the variables between the control and snorer groups, including age, body mass index, the difference in the largest oropharyngeal area, the difference in the smallest oropharyngeal area, the average difference between the largest and the smallest oropharyngeal area and thickness of the parapharyngeal and subcutaneous fat.
Table 1 shows the results of the variables between the control and snorer groups. Mean body mass index was 25.0±0.7 in control subjects and 28.2±0.7 in snorers. Although mean body mass index of the two groups revealed no obesity, the conventional measure of body mass index was significantly higher in the snorers (p < 0.05). The difference in the smallest oropharyngeal airway area between snorers and controls was statistically significant (p < 0.01). The difference in the largest oropharyngeal airway area between snorers and controls was not statistically significant (p > 0.05). The average difference between the largest and the smallest oropharyngeal areas was 52.1±32.6 mm2 in control group and 87.6±59.8 mm2 in snorer group. The difference was statistically significant (p < 0.05).
There was no significant difference in the total subcutaneous fat width and total parapharyngeal fat width between snorers and control subjects (p > 0.05).
Snoring is easily recognized as an unpleasant low-frequency noise that is accompanied by the vibration of the upper airway. The physical examination for this condition is non-specific, and it is usually limited to head and neck. There are some useful markers for snoring such as generalized body obesity, a short and fat neck, nasal polyps, septal deviation, nasal turbinate hypertrophy and evidence of previous nasal fractures (1). Abnormalities of upper airway anatomy in snorers have been demonstrated in many different investigations by using a variety of techniques such as lateral cephalography, awake endoscopy, awake endoscopy with the Muller manoeuvre, endoscopy during sleep, endoscopy with nasal continuous positive airway pressure during sleep, fluoroscopy, CT scanning, MR scanning, manometry, and acoustic reflections (1, 10, 11). The fundamental abnormality in patients that snore is still unclear. Whether it is due to anatomic or functional abnormalities is a subject of continuing investigation.
Many investigators have examined cross-sectional areas at various levels in the upper airway, and most of them have found smaller cross-sectional areas at several levels in the pharynx (13-16). The levels of occlusion and narrowing of the pharyngeal airways of patients with snoring and obstructive sleep apnea are variable, with the oropharynx being the most commonly affected part (11-15). In our study, the mean difference between the largest and the smallest area of the oropharynx in the snorers was significantly higher than the controls (p < 0.05), and in fact, the difference in the smallest oropharyngeal airway area between the snorers and controls was significant (p < 0.01).
Obesity is one of the risk factors for snoring and sleep-disordered breathing, and investigators have noted that body mass indexes were significantly greater in snorers (9, 18-20). Airway narrowing in the apneic or snoring patients was predominantly in the lateral dimension (10, 12, 19, 21-23). Some investigators have recently demonstrated that the increased lateral size of fat pads in snoring and apneic patients may help to explain why the apneic airway is compromised laterally (9, 12, 21, 24). Another investigators have observed that there was no abnormal collection of fat density adjacent to the pharyngeal airway and the size of fat pads was found to be normal on CT (22). The correlation between the BMI and the smallest airway area was not significant as well (19).
In our study, although there were statistically significant correlations in the oropharyngeal airway area measurements (the largest minus smallest area) and body mass indexes, there were no significant differences in fatty tissue thickness between the snorers and control groups. Our results show that airway narrowing does not result from the augmentation of the surrounding fat tissue in snorers, despite the fact that the snorers have slightly (but not significantly) increased fat deposition adjacent to the oropharynx. Mortimore and associates (24) supposed that greater fat deposition anterolateral to the upper airway in the snorers might predispose them to the airway narrowing and collapse during sleep. If that speculation is true, the maximum airway cross-sectional area as well as the minimum airway area also must be significantly smaller in the snorers than in the controls due to increased fat deposition. In contrast, in our series, the maximum airway area was not significantly different between the snorers and controls. Unfortunately, the measurements of the maximal airway area were lacking in the study of Mortimore et al. (24). Furthermore, the methods of measuring the airway area used in their study were somewhat questionable. The airway area changes from maximum to minimum even in one cycle of respiration. They obtained 20 slices from different levels during a total scanning time of 6 minutes (24). So, the slices obtained in their study might not include the actually smallest oropharyngeal airway area.
In conclusion, our study shows that the fat deposition in snorers is not an important factor predisposing the upper airway to narrowing. Further studies are warranted to investigate other factors that could be involved in snoring, such as lateral pharyngeal walls, reduction of muscle thickness, and disability of the pharyngeal muscles, soft palates, bony structures or tongue.
Figures and Tables
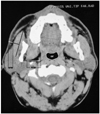 | Fig. 1Measurements of the thickness of parapharyngeal and subcutaneous fat.
(SFW: Subcutaneous fat width, PFW: Parapharyngeal fat width, OF: Oropharyngeal airway area)
|
References
1. Hoffstein V. Snoring. Chest. 1996. 109:201–222.
2. Block AJ, Boysen PG, Wynne JW, Hunt LA. Sleep apnea, hypopnea and oxygen desaturation in normal subjects. A strong male predominance. N Engl J Med. 1979. 300:513–517.
3. Wetter DW, Young TB, Bidwell TR, Badr MS, Palta M. Smoking as a risk factor for sleep-disordered breathing. Arch Intern Med. 1994. 154:2219–2224.
4. Palomaki H, Partinen M, Erkinjuntti T, Kaste M. Snoring, sleep apnea syndrome, and stroke. Neurology. 1992. 42:75–82.
5. Lindberg E, Janson C, Gislason T, Svardsudd K, Hetta J, Boman G. Snoring and hypertension: a 10 year follow-up. Eur Respir J. 1998. 11:884–889.
6. Jennum P, Hein HO, Suadicani P, Gyntelberg F. Cardiovascular risk factors in snorers. A cross-sectional study of 3,323 men aged 54 to 74 years: the Copenhagen Male Study. Chest. 1992. 102:1371–1376.
7. Suratt PM, Dee P, Atkinson RL, Armstrong P, Wilhoit SC. Flouroscopic and computed tomographic features of the pharyngeal airway in obstructive sleep apnea. Am Rev Respir Dis. 1983. 127:487–492.
8. Remmers JE, Anch AM, deGroot WJ. Respiratory disturbances during sleep. Clin Chest Med. 1980. 1:57–71.
9. Welch KC, Foster GD, Ritter CT, et al. A novel volumetric magnetic resonance imaging paradigm to study upper airway anatomy. Sleep. 2002. 25:532–542.
10. Ayappa I, Rapoport DM. The upper airway in sleep: physiology of the pharynx. Sleep Med Rev. 2003. 7:9–33.
11. Faber CE, Grymer L. Available techniques for objective assessment of upper airway narrowing in snoring and sleep apnea. Sleep Breath. 2003. 7:77–86.
12. Schwab RJ, Gefter WB, Hoffman EA, Gupta KB, Pack AI. Dynamic upper airway imaging during awake respiration in normal subjects and patients with sleep disordered breathing. Am Rev Respir Dis. 1993. 148:1385–1400.
13. Brown IG, Zamel N, Hoffstein V. Pharyngeal cross-sectional area in normal men and women. J Appl Physiol. 1986. 61:890–895.
14. Haponik EF, Smith PL, Bohlman ME, Allen RP, Goldman SM, Bleecker ER. Computerized tomography in obstructive sleep apnea.Correlation of airway size with physiology during sleep and wakefulness. Am Rev Respir Dis. 1983. 127:221–226.
15. Rivlin J, Hoffstein V, Kalbfleisch J, et al. Upper airway morphology in patients with idiopathic obstructive sleep apnea. Am Rev Respir Dis. 1984. 129:355–360.
16. Bradley TD, Brown IG, Grossman RF, et al. Pharyngeal size in snorers, nonsnorers, and patients with obstructive sleep apnea. N Engl J Med. 1986. 315:1327–1331.
17. Chaban R, Cole P, Hoffstein V. Site of upper airway obstruction in patients with idiopathic obstructive sleep apnea. Laryngoscope. 1988. 98:641–647.
18. Ah-See KW, Stewart M, Banham SW, Robinson K, Carter R, Wilson JA. Systematic analysis of snoring in woman. Ann Otol Rhinol Laryngol. 1998. 107:227–231.
19. Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patiends with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995. 152:1673–1689.
20. Mayer P, Pepin JL, Bettega G, et al. Relationship between body mass index, age and upper airway measurements in snorers and sleep apnoea patients. Eur Respir J. 1996. 9:1801–1809.
21. Horner RL, Mohiaddin RH, Lowell DG, et al. Sites and sizes of fat deposits around the pharynx in obese patients with obstructive sleep apnoea and weight matched controls. Eur Respir J. 1989. 2:613–622.
22. Shelton KE, Woodson H, Gay S, Suratt PM. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis. 1993. 148:462–466.
23. Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003. 168:522–530.
24. Mortimore IL, Marshall I, Wraith PK, Sellar RJ, Douglas NJ. Neck and total body fat deposition in nonobese and obese patients with sleep apnea compared with that in control subjects. Am J Respir Crit Care Med. 1998. 157:280–283.




