Multidetector CT urography (MDCTU) offers several advantages for imaging of the urinary tract: single breath-hold coverage of the entire urinary tract with absence of respiratory mis-registration, rapid imaging with optimum contrast medium opacification and reduced partial-volume effect as appropriate slices can be selected from the volumetric data. In addition, acquisition of multiple thin overlapping slices provides excellent two-dimensional (2D) and three-dimensional (3D) reformations, and facilitates virtual cystoscopy (1, 2). These advantages have established MDCTU as a compelling alternative to excretory urography and ultrasonography (US) in the evaluation of the patient with hematuria. The increased utilization of cross-sectional imaging in the investigation of the urinary tract has led to a marked reduction in number of excretory urograms being performed annually, particularly in the United States (3, 4). The number of excretory urograms currently being performed per year in the U.S. has declined appreciably from over a million examinations per annum in 1975 to 600,000 examinations per annum (3). Indeed, in many academic medical centers, MDCTU has replaced excretory urography as the "core" imaging study for investigation of hematuria. MDCTU has the potential to stand alone as a comprehensive "one-stop" test for imaging the upper and lower urinary tract. It is especially suitable for patients presenting with hematuria where the urinary tract must be assessed for both stone disease and neoplasms of the kidney and/or urothelium (5, 6). For the investigation of hematuria, most authors agree that excretory urography cannot approach the sensitivity of ultrasound or CT in the evaluation of the renal parenchyma and needs to be supplemented by either cross-sectional modality in order to exclude renal parenchymal abnormalities. This review article describes the techniques of MDCTU and discusses the benefits and limitations of MDCTU in the evaluation of the patient with hematuria.
Technical aspects
Imaging Protocol
Most medical institutions employ a three-phase MDCTU protocol for the evaluation of patients with hematuria. Most three-phase MDCTU protocols comprise an initial non-contrast phase to detect urinary tract calculi and a second phase, i.e. the nephrographic phase, which is acquired following a delay of 90-100 seconds after administration of 120 ml of intravenous iodinated contrast, to evaluate the renal parenchyma. This is followed by the pyelographic phase taken 5-10 minutes following contrast administration, to evaluate the urothelium from the pelvicaliceal system to the bladder. These three-phase protocols are used at most institutions as they allow a thorough evaluation of the urinary tract for the most common causes of hematuria i.e. urinary tract calculi, renal neoplasms and urothelial tumors. However, this approach has three main disadvantages: 1) it involves a very high radiation dose and 2) it is time consuming, a factor which can impact significantly on increasing radiological daily workloads, 3) it increases the number of images for review by the radiologist.
To address these disadvantages of three-phase technique, a two-phase protocol has been developed for the evaluation of patients with hematuria (7). In this article, we will describe the two-phase technique and discuss its advantages and disadvantages in imaging the patient with hematuria. As part of a two-phase MDCTU protocol, a non-contrast scan is initially obtained from the top of the kidneys to the base of the bladder. The non-contrast scan is performed without intravenous and oral contrast to exclude urinary tract calculi. The omission of oral contrast is to facilitate 3D reformations of the contrast-filled ureters and collecting systems if they are considered necessary at the end of the study. An unenhanced CT without oral or intravenous contrast scan has been shown to be the most sensitive test in the evaluation of the urinary tract for calculi (8). For those patients in whom the indication for MDCTU is microscopic hematuria, the following policy with regard to requirement for contrast-enhanced study (Fig. 1) is used. The requirement for contrast-enhanced study is dependent on the findings on the unenhanced study and on the age and clinical presentation of the patient. If urinary tract calculi are detected on the non-contrast scan in a patient less than 40 years, the study is terminated. However, if urinary tract calculi are not detected in patients less than 40 years old or if findings do not correlate with clinical findings, a contrast enhanced scan is performed. The rationale for not giving intravenous contrast to patients less than 40 years is the negligible incidence of urological malignancy reported in patients in this age group (9, 10). A contrast-enhanced "nephropyelographic phase" scan is obtained in all patients with hematuria, greater than 40 years old, and in all patients with macroscopic hematuria regardless of the findings on initial unenhanced study. This policy is based on the current guidelines of the American Urological Association Best Practice Policy (8, 10).
The following protocol is performed in those patients who require "nephropyelographic phase". The patient is taken off the CT table after the initial non-contrast examination, and 30 cc of non-ionic contrast material are infused. As with excretory urography, the entire length of a normal ureter is seldom opacified at MDCTU. To optimally opacify and distend the ureters, 100 cc of saline is infused immediately after injection of the 30 cc bolus of non-ionic contrast (11, 12). As will be discussed later in this review, it is currently unclear, whether MDCTU offers as thorough an evaluation of the urothelium as excretory urography. Nevertheless, most authors agree that a complete evaluation of the urothelium by MDCTU is dependent on optimum ureteric distension and opacification and this motivates the administration of 100 cc of saline intravenously. McTavish et al. have reported improved ureteric opacification and distension following the administration of 250 cc of saline (12). It has been observed that less significant improvement in ureteric opacification and distension during MDCTU is observed following the administration of 100 cc of saline intravenously (11). With regard to the utilization of abdominal compression for visualization of the urinary tract, two recent papers have demonstrated an increase in renal collecting system and ureteric distension following the application of abdominal compression (13, 14).
After 10-15 minutes, the patient is again placed on the CT table and a dynamic contrast-enhanced study is performed in the prone position, following the administration of an additional 100 cc of non-ionic contrast material (300 mg/ml injected at 2 cc/s) following a 100 seconds delay to allow renal parenchymal examination in the nephrographic phase. Thus, in a single "nephropyelographic phase" acquisition, the renal parenchyma is assessed in the nephrographic phase and the collecting system, ureters and bladder are assessed in the pyelographic phase. The nephrographic phase has been shown to have higher sensitivity for detecting renal masses and in combination with unenhanced images is also effective for renal mass characterization (5, 15). Recent reports in the literature have suggested that streak artifacts from contrast medium in the calyces may obscure small renal masses in the pyelographic phase (16). Although this is a potential disadvantage of the two-phase protocol, which evaluates the renal parenchyma while contrast is present in the pelvicaliceal system, problems with streak artifact are rarely significant. This may be explained by the fact that the collecting system is opacified with only 30 cc of contrast thus reducing the quantity of contrast in the calyces and collecting system. It is important to interpret the pyelographic phase images with different window and level settings, particularly for evaluation of collecting system as subtle filling defects caused by urothelial lesions, blood clots or non-radiopaque calculi may be obscured by dense intraluminal contrast.
In suspected urolithiasis, contrast enhanced images are required occasionally, when a calcification is identified on un-enhanced images in the vicinity of the ureter or pelvicaliceal system, but cannot be definitively established as being intraluminal or extraluminal. This situation is most commonly encountered in the distal ureter, where non-obstructing calculi can be difficult to distinguish from phleboliths or vascular calcifications, and in the kidneys where vascular calcifications can mimic renal calculi on non-enhanced images.
Scanning Parameters
CT scanning is performed extending from the top of the kidneys to the base of the bladder in an approximately 20 second breath-hold using a 4-channel multislice helical scanner. Images are acquired with a 2.5 mm detector configuration and a non-overlapping slice-pitch of 1.5:1 (table speed 15 mm/rotation). For diagnostic evaluation, contiguous axial images are reconstructed with 5-mm slice thickness. When needed, 2.5 mm thin slices (slice profile 3.2 mm at FWHM) with 50% overlap are obtained for reconstructing coronal and sagittal images of the ureters. Thinner slices are especially important in evaluation of renal vessels, and small and subtle renal abnormalities. The scan parameters for MDCTU have been summarized in table 1.
Three-dimensional (3D) reconstruction
Three-dimensional (3D) reconstruction of the image data includes thick and thin slab coronal and sagittal maximum-intensity-projection for kidneys, ureters and urinary bladder. When MDCTU was introduced initially, the majority of studies were supplemented with 3D reconstructions. The 3D reconstructions aided in convincing urologists of the benefits of this technique over excretory urography as it allowed them to view images in the coronal plain similar to excretory urography images.
In addition, for radiologists, experience in the characterization of certain pathologies particularly those of the renal papillae, such as renal tubular ectasia and papillary necrosis had been gained by evaluation of excretory urography images, which were acquired in the coronal plane and initially difficulties were encountered in characterizing these pathologies on transverse plane images (13). 3D reformations particularly in coronal plane are very useful for characterizing these conditions (13). Caoili et al. have reported that 3D reconstructions are a useful aid to radiologists and urologists as a "bridge" between excretory urography data and transverse MDCTU data (13). We also concur that as additional experience is gained with MDCTU, most urinary tract pathologies will be characterized in the transverse plane, and 3D reformations may no longer be necessary. Furthermore, it should also be emphasized that 3D reformations suffer the same disadvantages as excretory urography if the transverse images are not reviewed in association. Many ureteral and bladder wall abnormalities are frequently detected on transverse images and can be missed on 3D reformats. (13).
Comparison of MDCTU and MR urography
One of the main advantages of MDCTU in the evaluation of the urinary tract for causes of hematuria, is its ability to display the entire urinary tract, including renal parenchyma, pelvicaliceal systems, ureters and the bladder using a single imaging test. The alternative imaging studies including ultrasonography, excretory urography, and nuclear medicine alone do not offer equivalent coverage. Magnetic resonance urography (MRU) is the only other alternative study, which can image all the anatomic components of the urinary tract in a single test (17). MRU has advantages over MDCTU including the ability to image the pelvicaliceal systems without intravenous iodinated contrast agents using heavily T2 weighted ultrafast sequences (17). Another advantage of MRU is that the significant radiation dose associated with the other modalities is avoided (17). However, contrast is usually required to evaluate the renal parenchyma especially for renal masses. The main disadvantages of MR urography, which have hindered its widespread usage in the evaluation of the urinary tract, is its inability to reliably detect urinary tract calcifications, calculi and air (17); limited availability in comparison to MDCTU; and limited experience in interpretation of images (17).
MDCTU is a versatile imaging test, which can clearly demonstrate urinary tract anomalies, inflammatory processes, calculus diseases, and benign and malignant neoplasms. Consequently, it is being increasingly recommended as a first line of investigation in the patients with hematuria.
Congenital anomalies and normal variants
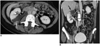
Most congenital anomalies of urinary tract can be appreciated with MDCTU (Fig. 2). Congenital anomalies of renal position, number and form are well depicted by MDCTU (1). With optimum opacification of ureters, partial and complete duplication of the collecting system can be seen on axial source images. Improved z-axis resolution are a welcome consequence of MDCTU and aid in obtaining diagnostic quality three-dimensional reconstructions, particularly in coronal plane. These factors have improved our ability to thoroughly evaluate the urinary tract for variant anatomy. 3D reconstructions can be very useful in the characterization of urinary tract anomalies such as ureteral duplication and ectopic ureter or ureteroceles (16). An advantage of MDCTU in this clinical setting is that MDCTU can depict not only opacified ureters but also unopacified ureters, which cannot be visualized on excretory urography (18). Variant anatomy can impact on the performance of urological endoscopy and surgery and also on percutaneous intervention. Detection and mapping of these anomalies is therefore important when planning these interventions and MDCTU improves our ability to accomplish this.
Urolithiasis
Urolithiasis is a common cause of hematuria and MDCTU accurately detects urinary tract calculi on the initial unenhanced study. Prior to the development of MDCTU, for patients presenting with acute flank pain or hematuria, the recommended diagnostic approaches included plain radiography, excretory urography, ultrasound and computed tomography used in various combinations. Conventional radiography had a sensitivity of only 60% in detecting urolithiasis and in combination with US, the sensitivity increased to 70% (13, 19). Although, excretory urography is reasonably accurate for detecting urinary tract stones, some reports suggest that it fails to demonstrate calculi in up to 48% of cases (20, 21)
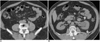
Nearly all stones, including those containing uric acid and those located in unusual positions such as caliceal diverticulum (Fig. 3) are detectable by CT (5). An unenhanced study is highly sensitive and accurate in diagnosing obstruction secondary to ureteric calculi. It is more accurate than excretory urography in demonstrating the presence, size and location of urinary tract calculi. The diagnosis of obstructing urinary tract calculi is usually confirmed by the detection of the secondary signs of obstruction. Presence of "soft tissue rim sign," a circumferential rim of soft-tissue attenuation surrounding an abdominal or pelvic calcification, is a strong indicator that a calcification along the course of the ureter is a calculus (Fig. 4) (22, 23). Similarly, a "comet-tail sign" representing a linear or curvilinear soft-tissue structure extending from an abdominal or pelvic calcification, is an important indicator that a suspicious calcification represents a phlebolith, while its absence suggests indeterminate calcification (23, 24). Coll and colleagues have documented the relationship of spontaneous passage of ureteral calculi to stone size and location using unenhanced helical CT (25).
The non-contrast component of the MDCTU is essential as opacified ureters may hide calculi. In examining the opacified ureter, the use of bone windows is suggested, as it increases conspicuity of urinary tract calcifications and improves the detection of urinary tract filling defects.
For the exclusion of urolithiasis, contrast-enhanced scans are only indicated when uncertainty exists as to whether calcifications reside within the urinary tract. Uncertainties are most common in the kidney where parenchymal and vascular calcifications can be misclassified as calculi and in the pelvis where phleboliths can be confused with ureteric calculi. Unenhanced CT allows for relatively easy detection of ureteric course, non-obstructing calculi are more easily detected by MDCTU than on excretory urography. Occasionally, the confirmation of the presence of non-obstructing calculi requires a contrast-enhanced study. In contrast, one of the disadvantages in the evaluation of acute urinary tract obstruction with excretory urography, is the difficulty in detecting the site of urinary tract obstruction. This often necessitates delayed radiographs at intervals up to 24 hours. In this scenario, MDCTU has advantages over excretory urography as the entire unopacified ureter can usually be "tracked" to the site of obstruction (18).
Urinary Tract Neoplasms
Neoplasms of the urinary tract are a common etiology for hematuria and their detection can frequently be challenging, occasionally requiring multiple diagnostic tests. CT has been shown to be more accurate in the detection of parenchymal masses compared to ultrasound or excretory urography with sensitivities of 94% reported compared to 67% and 79% for excretory urography and ultrasound respectively (5). CT can detect up to 47% of masses measuring 5mm and 75% of masses measuring 10-15 mm in diameter (26). In fact, almost 30% of malignant renal masses are now detected incidentally during routine abdominal US or CT and, as they are usually smaller, can be amenable to nephron-sparing surgery with similar survival rates to those achieved with radical nephrectomy (27, 28).
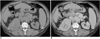
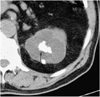
The CT characteristics of a simple renal cyst requires confirmation of water density throughout the lesion, sharply defined margins with no discernible wall and unequivocal absence of contrast enhancement. The Bosniak system was described in an effort to facilitate the distinction of benign cystic lesions and cystic neoplasms, and to guide the management of cystic renal masses (Fig. 5) (29). Pseudoenhancement has been described as a factor, which resulted in cystic lesions being wrongly categorized as solid lesions. Recent phantom studies have suggested that pseudoenhancement is a greater problem with small lesions and is more commonly seen with helical and multidetector helical CT scans (30). The use of thin section acquisition combined with thinner reformatting can reduce (15, 31) the impact of volume averaging and allows more accurate Hounsfield values to be calculated, thus reducing the likelihood of pseudoenhancement.
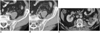
Transitional cell carcinoma is the commonest malignant neoplasm of the urothelium. In the early stages, these neoplasms are seen as subtle filling defects or focal mural thickening (Fig. 6). The ability of MDCTU to detect urothelial tumors in the renal collecting system or in the ureter has not been thoroughly evaluated or established in the literature. Caoili et al. reported that 15 of 16 (6 in the renal pelvis and ureters, and 9 in the bladder) proven urothelial malignancies were detected by CT urography (13). Many urologists believe that excretory urography is still the "gold-standard" for evaluating the urothelium. However despite this, excretory urography has been reported in the literature to have detection rates for urothelial neoplasms of only 43-64% (13).
Based on the accruing experience with MDCTU, it is now believed that adequate distension and opacification of the ureter and pelvicaliceal system are fundamental factors in the thorough evaluation of the urothelium (Fig. 7). Urothelial pathology can be difficult to appreciate even with optimal ureteric distension and opacification at standard soft tissue windows. Viewing the opacified ureters and pelvicaliceal system at wider window settings such as "bone windows" aids in identifying subtle filling defects in patients with hematuria and in distinguishing ureteral neoplasms from other filling defects. Coronal reconstructions of the ureters provide anatomic views of the urinary tract similar to those seen at excretory urography, with equivalent anatomic detail to excretory urograms. 3D reconstructions can also demonstrate the longitudinal extension of a lesion, and can evaluate for the presence of multicentric tumors. The urinary tract distal to an obstructing lesion is also well demonstrated, thus overcoming the limitations of excretory urography in a non-functioning kidney with obstructive disease (32). Other advantages of MDCTU over excretory urography include identification and characterization of the causes of ureteric obstruction including short segment malignant strictures with associated mural thickening, retroperitoneal masses and lymphadenopathy, retroperitoneal fibrosis, benign ureteric strictures and iatrogenic causes such as post hysterectomy and colectomy injuries (33, 34).
As with other urinary tract tumors, assessment of bladder tumors requires contrast-enhanced examination with optimum distention and opacification for detection of urinary bladder wall abnormalities. Occasionally the two-phase MDCTU technique can be modified in cases of suspected or known bladder neoplasms with an additional set of bladder images 5-10 minutes following the nephropyelographic phase to obtain a more densely opacified and more distended bladder (Fig. 8). Non-contrast images of the bladder are again very important in order to detect focal areas of mural calcification and focal thickening of bladder wall, which are usually suspicious for bladder malignancy. Virtual CT cystoscopy evolved with CT colonography as a means of evaluating the bladder urothelium although it is much less widely utilized in clinical practise. The additional information acquired at virtual CT cystoscopy can potentially aid in the planning of cystoscopy and cystoscopic resection of bladder tumors. For polypoidal tumors, reports in the literature have suggested that virtual endoscopy of urinary bladder has equivalent accuracy to conventional cystoscopy, and may be very useful in the follow-up of patients following cytoscopic resection or other local treatments of bladder tumors thus reducing the costs and morbidity associated with conventional cytoscopy. Kim et al. recently reported sensitivity and specificity of 95% and 87% of virtual cystoscopy for identifying bladder lesions (2). Although virtual cystoscopy may not provide any information about flat mucosal lesions, further trials will reveal whether color mapping of the bladder wall is feasible in the identification of small or sessile tumors and expand the role of virtual cystoscopy in follow-up of polypoidal lesions post local resection (34).
Urinary Tract Infections
Urinary tract infections are a common cause of hematuria. However, in the majority of cases, uncomplicated urinary tract infections, including acute pyelonephritis are adequately diagnosed by microbiologic analysis of the urine and can be managed without the need for cross-sectional imaging (35). However, severe sepsis with accompanying pyuria is sometimes an indication for MDCTU, particularly for the exclusion of pyonephrosis or renal abscess. In addition, acute pyelonephritis or renal abscess is occasionally discovered during MDCTU performed for investigation of hematuria and the typical findings are discussed below. Acute pyelonephritis is usually well characterized by MDCTU with findings of a "striated nephrogram" in a swollen kidney and stranding of the perinephric fat. Occasionally there can be thickening of the pelvicaliceal wall, which can also show increased mucosal enhancement (35-39). In acute pyelonephritis, MDCTU typically demonstrates solitary or multifocal hypodense areas with obliteration of normal corticomedullary differentiation. Parenchymal abnormalities are best demonstrated on CT images obtained in the nephrographic phase while excretory phase acquisitions are better for diagnosing renal abscesses than images acquired in the corticomedullary or nephrographic phases (38). The nephropyelographic phase of the two-phase protocol combines the advantages of both these phases in a single acquisition.
Focal low attenuation regions suggest renal abscess. The finding of gas within a parenchymal fluid collection or the renal collecting system is consistent with more severe infection. Xanthogranulomatous pyelonephritis is a severe form of renal infection associated with long-term renal obstruction and infection. CT scan demonstrates a heterogeneous non-enhancing mass in a hydronephrotic nonfunctioning kidney with density of -15 to +15 HU. In severe cases, the mass extends beyond the confines of the kidney to involve adjacent organs (Fig. 9). A large staghorn calculus is usually demonstrated within the collecting system.
In patients with renal papillary necrosis, CT may demonstrate small kidneys, "ring shadows" in the medullae, contrast filled clefts in the renal parenchyma and filling defects in the renal collecting systems and ureters. Caoli et al have reported that the diagnosis of caliceal abnormalities such as papillary necrosis or medullary sponge kidney can be difficult to make on axial MDCTU images and that coronal images can be helpful in this respect (13). However, Lang et al subsequently reported that renal and medullary necrosis can be detected at an earlier stage by MDCTU than be excretory urography (40).
Non-Tumorous Renal Lesions
In the evaluation of patients with hematuria, contour abnormalities in the kidneys are incidentally seen by ultrasound and it can be difficult to differentiate the common causes of these contour abnormalities, which include hypertrophied column of Bertin, dromedary hump, focal scarring or renal masses. The use of coronal or sagittal reconstructions can be useful in the evaluation of these contour abnormalities to exclude a more ominous lesion.
Caliceal diverticulum may be seen on a non-contrast study, as a loculated pocket of "milk of calcium" in a peripheral location. On delayed phase images, this area fills with contrast and a peripheral contrast-filled "pouch" can be identified extending from the periphery of the collecting system into the adjacent renal parenchyma. Similarly, differentiation of hydronephrosis from parapelvic cysts can also be easily established in the nephropyelographic phase of MDCTU.
As described previously, experience in the characterization of certain pathologies, particularly those of the renal papillae, such as renal tubular ectasia and papillary necrosis, had been gained by evaluation of excretory urography images and initially difficulties were encountered in characterizing these pathologies on transverse plane MDCTU images. 3D reformations were found to be very useful for characterizing these conditions (13). As additional experience is gained allowing most urinary tact pathologies to be characterized in the transverse plane, 3D reformations may no longer be necessary (13).
Bladder Tumors
Evaluation of the bladder by MDCTU requires pre-and post contrast studies. The pre-contrast study is useful as calcifications within the bladder wall or lumen are easily detected. However, bladder masses frequently cannot be detected on non-contrast images. CT scanning is performed in the prone and supine positions to confirm that the calcifications are within the lumen rather than in the bladder wall or in the case of ureteral calculi, impacted at the ureterovesical junction. Diffuse calcification within the bladder wall can result from cyclophosphamide induced cystitis, schistosomiasis, or tuberculosis. Focal bladder wall calcification can occur with transitional cell or squamous cell carcinoma of the bladder. Focal areas of bladder wall thickening suggest bladder carcinoma particularly when it is associated with increased enhancement of the bladder wall at that point. Scanning of a fully distended contrast-filled bladder may demonstrate these tumors as filling defects. Virtual cystoscopy is another evolving dimension in the CT evaluation of patients with hematuria with sensitivities of 90% reported for bladder lesions seen by cystoscopy (34).
Incidental Findings
In the evaluation of the hematuria patient, MDCTU compared to excretory urography has the obvious advantage of detecting pathology outside of the urinary tract, the majority of which cannot be detected at excretory urography. However, a disadvantage of this is that many incidental findings at MDCT are of uncertain clinical significance, causing unnecessary stress for the patient and precipitating additional diagnostic tests.
Future Applications of MDCTU
At most US medical institutions, MDCT has replaced excretory urography as the diagnostic imaging study of choice for the evaluation of patients with ureteric colic or with suspected urolithiasis. For patients with hematuria, MDCTU is also considered the "gold standard" imaging test for the evaluation of the renal parenchyma for renal masses. However, the "last frontier" for MDCTU before it is universally accepted, as a "one stop" imaging test in the evaluation of hematuria is that its sensitivity, specificity and overall diagnostic accuracy in the evaluation of urothelial neoplasms must be scientifically proven. Until this is established in a prospective trial, many urologists and radiologists may continue to utilize excretory urography for evaluation of the urothelium in patients with hematuria.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download