Abstract
Objective
To determine the potential value of distributional-phase T1-weighted ferumoxides-enhanced magnetic resonance (MR) imaging for tissue characterization of focal liver lesions.
Materials and Methods
Ferumoxides-enhanced MR imaging was performed using a 1.5-T system in 46 patients referred for evaluation of known or suspected hepatic malignancies. Seventy-three focal liver lesions (30 hepatocellular carcinomas [HCC], 12 metastases, 15 cysts, 13 hemangiomas, and three cholangiocarcinomas) were evaluated. MR imaging included T1-weighted double-echo gradient-echo (TR/TE: 150/4.2 and 2.1 msec), T2*-weighted gradient-echo (TR/TE: 180/12 msec), and T2-weighted turbo spin-echo MR imaging at 1.5 T before and after intravenous administration of ferumoxides (15 mmol/kg body weight). Postcontrast T1-weighted imaging was performed within eight minutes of infusion of the contrast medium (distributional phase). Both qualitative and quantitative analysis was performed.
Results
During the distributional phase after infusion of ferumoxides, unique enhancement patterns of focal liver lesions were observed for hemangiomas, metastases, and hepatocellular carcinomas. On T1-weighted GRE images obtained during the distributional phase, hemangiomas showed a typical positive enhancement pattern of increased signal; metastases showed ring enhancement; and hepatocellar carcinomas showed slight enhancement. Quantitatively, the signal-to-noise ratio of hemangiomas was much higher than that of other tumors (p < .05) and was similar to that of intrahepatic vessels. This finding permitted more effective differentiation between hemangiomas and other malignant tumors.
Imaging work-up of the liver is performed primarily to screen patients with known primary malignancies or those at high risk, or to characterize lesions detected with other modalities (1). Superparamagnetic iron oxides (SPIOs), such as ferumoxide, are reticuloendothelial-specific contrast agents taken up primarily by the Kupffer cells of the liver and used to improve the detection of focal liver lesions at MR imaging (2-6). After SPIO administration, the liver parenchymal signal seen at T2- and T2*-weighted imaging increases liver-to-lesion contrast and potentially improves the detection of focal liver lesions (2-3). It has been shown that for the detection of focal liver lesions, SPIO-enhanced MR imaging is substantially more sensitive than CT or non-enhanced MR imaging, and at least as accurate as CT during arterial portography (1, 4, 6). In previous studies of ferumoxides-enhanced MR imaging, T2*-weighted gradient-recalled echo (GRE) and T2-weighted turbo spin-echo (TSE) sequences were used and are considered the preferable techniques for SPIO-enhanced MR imaging.
In determining an accurate treatment plan, the characterization of focal hepatic lesions is as important as their detection. Furthermore, since up to 50% of lesions less than 15 mm in diameter may be benign, lesion characterization is of special importance in patients with a primary hepatic malignancy (7). Although ferumoxides-enhanced MR imaging may increase the detectability of focal liver tumors, their characterization may be problematic because their appearance can be modified by ferumoxides (8-11). Some reports have claimed that tissue characterization improves with T1-weighted imaging after the administration of SPIOs, using their R1 relaxivity (8-10, 12, 13); in those studies, images were usually acquired one hour after ferumoxides administration ended. However, the plasma half-life of ferumoxides is reported to be less than 10 mins (9, 14), and to observe their T1 effect, the earlier acquisition of images, during the distributional phase (less than 10 minutes after administration) and using a T1-weighted sequence with minimum echo time, may be helpful and may provide additional information for tissue characterization of focal liver lesions.
The purpose of this study is to assess the value of ferumoxides-enhanced T1-weighted MR imaging performed during the distributional phase for tissue characterization of focal liver lesions.
Our study population comprised 46 patients (35 men and 11 women) with a mean age of 58.5 (range, 52-72) years. Informed consent was obtained from each. Seventy-three lesions were detected and included 30 hepatocellular carcinomas (HCC) in 20 patients, 12 metastases in six, 15 cysts in nine, 13 hemangiomas in eight, and three cholangiocarcinomas in three. In each subject, between one and four lesions were present. Two had two types of lesion and one had three types. The lesions were 0.5-7 (mean 3.4±1.8) cm in diameter.
Diagnosis of the various lesions was established as follows. That of HCC was based on the findings of percutaneous biopsy (n=7), examination of a surgical specimen (n=3), or was on the basis of typical clinical and laboratory findings in combination with evidence of disease progression, as depicted at follow-up imaging (n=10). Liver metastases were diagnosed on the basis of histological findings in the primary tumor and rapid progression of the lesion, as depicted at serial follow-up imaging (n=2) or by examining percutaneous needle biopsy specimens (n=4). Liver metastases were related to the following histologically proven primary tumors: carcinoma of the colon (n=3), stomach (n=2), and lung (n=1). Confirmation of cholangiocarcinomas was based on the histological findings (n=3), while hemangiomas were diagnosed on the basis of their typical appearance at triple-phase helical computed tomography and dynamic MR imaging performed sequentially over a five-minute period following the injection of iodinated contrast medium or gadolinium chelates, and the absence of growth during a follow-up period of at least six months (15-18). For liver cysts, diagnosis was based on established criteria, as observed at contrast-enhanced CT, ultrasound and unenhanced MR imaging, and during a clinical follow-up period of at least six months (15, 16).
Images were obtained with a 1.5 T scanner (Symphony; Siemens Medical Systems, Erlangen, Germany) using a body phased-array coil. All patients underwent unenhanced and ferumoxides (Feridex I.V.; Advanced Magnetics, Cambridge, Mass., U.S.A.)-enhanced MR imaging using the following sequences: respiratory-triggered, T2-weighted turbo spin-echo (TSE); breath-hold T2*-weighted gradient-recalled echo (GRE); breath-hold T2-weighted TSE; and breath-hold T1-weighted double-echo GRE images (TE 4.2/ 2.1 msec).
For respiratory-triggered T2-weighted TSE imaging, the following parameters were used: a TR range/effective TE of 3500-5600/80, an echo-train length of 15, three signal averages, and a matrix of 192×256. Those for breath-hold T2-weighted TSE included a TR/TE of 2500/100, a flip angle of 150, and a matrix of 132×256, while for breath-hold T2*-weighted gradient-echo imaging, a TR/TE of 180/12, a flip angle of 30°, and a matrix of 132×256 were used. One signal was acquired and two or three data acquisitions were performed. Breath-hold T1-weighted double-echo GRE images were obtained with a TR/TE 150/4.2 and 2.1, a 70° flip angle, one signal average, and a matrix of 256×132. For all sequences, imaging was performed in the axial plane with a section thickness of 7 mm, an intersection gap of 3 mm, and a rectangular field of view of 24-28×30-34 cm. The phase-encoding direction was anterior to posterior for all sequences.
Ferumoxides was administered at 15 µmol of iron per kilogram of body weight; the suspension was diluted in 100 mL of 5% glucose solution and administered intravenously at a rate of approximately 4 mL/min for 25 minutes. Postcontrast T1-weighted MR images were obtained not more than 10 minutes later (distributional phase), and postcontrast T2-weighted and T2*-weighted images were obtained 10-30 minutes after the end of contrast administration (accumulation phase).
Two radiologists experienced in abdominal MR imaging assessed the enhancement pattern of both precontrast and postcontrast (ferumoxides-enhanced) MR images. They had no knowledge of patient history or diagnosis, and reached their conclusion by consensus. For each lesion, signal intensity relative to hepatic parenchyma was recorded as hypo-, iso- or hyperintense. For hyperintense lesions showing enhancement at ferumoxides-enhanced T1-weighted imaging, the readers were asked to state whether or not the enhancement patterns were ring-shaped.
To assess the reliability of ferumoxides-enhanced T1-weighted imaging for the characterization of focal liver lesions, two radiologists who interpret MR images of the liver as part of their daily clinical and research practice, and who were blinded to the diagnosis and clinical history, reviewed two sets of images: a) postcontrast accumulation-phase T2-weighted and T2*-weighted (reading set A); and b) combined distributional-phase T1-weighted in- and opposed-phase and accumulation phase (reading set B). The images were randomized and presented at two sessions, with patient data masked. To minimize learning bias, the review sessions were held at two-week intervals. At each, the readers were asked to provide a diagnosis according to previously published established criteria, and a consensus was then reached (8-12, 14-16).
The diagnostic criteria for cyst, hemangioma, metastasis and HCC were as follows. Lesions showing no enhancement at postcontrast T1- and T2-weighted imaging were considered to be cysts; those showing positive enhancement similar to that of the intrahepatic vessels at T1-weighted and negative enhancement at T2-weighted imaging were classified as hemangiomas. Lesions in which ring enhancement was apparent at postcontrast-T1 weighted imaging were considered to be metastases, while those showing slight enhancement at T1-weighted and no enhancement at T2-weighted imaging were classed as HCCs. To assess the statistical significance of the differences between the reading sessions, the McNemar test was used.
The degree of enhancement of the liver, tumors, and the portal vein was calculated from signal intensity measurements before and after intravenous injection of ferumoxides. For a lesion and for normal liver parenchyma, operator-defined region-of-interest (ROI) SI measurements were obtained directly from the monitor. During the various imaging sequences, care was taken to place the ROI for the liver or portal vein in the same area, and for lesions with necrotic areas, care was taken to measure SI only in solid portions of the tumor. Whenever possible, an ROI of at least 35 mm2 was used; similar regions of interest were used for unenhanced and ferumoxides-enhanced images. For each image, background noise was measured ventral to the liver outside the patient along the direction of the phase-encoding gradient and included any ghosting artifacts that might have been propagated over the images. For each sequence, signal-to-noise ratios (SNRs) for lesions and liver were calculated using the following formula: SNR = SI / SD, where SI is the signal intensity of the lesion or liver parenchyma and SD is the standard deviation of background noise. In addition, contrast-to-noise ratios (CNRs) for lesion-to-liver and portal vein-to-liver were computed for each sequence as follows: CNRlesion = (SI lesion - SI liver) / SD, and CNRPV = (SI portal vein - SI liver) / SD. These values were negative for hypointense lesions and positive for hyperintense lesions.
One-way analysis of variance with pair-wise comparisons was used to determine the statistical significance of the differences between the ferumoxides-enhanced CNRs of each type of tumor, as seen at T1-weighted FLASH imaging. The post hoc Tukey test was used to determine which differences were statistically significant, indicated by a p value of less than 0.05.
Before ferumoxides administration, T1-weighted FLASH imaging showed that 61 of 70 lesions were hypointense relative to the liver. Seven HCCs were slightly hyperintense and two were isointense.
For each type of lesion, ferumoxides-enhanced T1-weighted double-echo GRE images obtained during the distributional phase demonstrated some degree of characteristic enhancement. All 13 hemangiomas showed positive enhancement at T1-weighted in- and out-of-phase GRE imaging (Fig. 1). Intrahepatic vessels were also hyperintense to the liver, with a degree of intensity similar to that demonstrated by hemangiomas. In all of these, a slight degree of signal intensity drop was noted, though for metastatic tumors, HCCs and cysts, this was not the case. Ferumoxides-enhanced T1-weighted double-echo imaging revealed no enhancement of cystic lesions, though at distributional-phase T1-weighted in- and out-of-phase imaging, peripheral rim enhancement was seen in nine metastases (75%) and two of three cholangiocarcinomas (67%) (Figs. 2, 3). In addition, T1-weighted dual-echo imaging of HCCs revealed slight enhancement in 24 lesions (80%), ring enhancement in two (6.7%), and no noticeable enhancement in four (13.3%).
In terms of the relative signal intensity of focal liver lesions observed at T1-weighted double-echo GRE imaging, out-of-phase images obtained using minimum echo time better differentiated hemangiomas from other tumors than did in-phase images (Table 1). Distributional-phase T1-weighted out-of-phase imaging showed that no hemangioma was hypointense, but 11 of 12 metastatic lesions (92%) and three cholangiocarcinomas (100%) were (Table 1). This same modality showed that 19 of 30 HCCs (63%) were hypointense, but nine HCCs seen as hyperintense at precontrast T1-weighted imaging were also seen as hyperintense on all ferumoxides-enhanced T1-weighted out-of-phase images because liver parenchyma showed a signal decrease after the infusion of ferumoxides (Fig. 4) (Table 1).
A summary of the mean signal-to-noise ratios of liver lesions, liver parenchyma, and the portal vein, as seen at T1-weighted imaging before and after ferumoxides enhancement, is shown in Table 2. The enhancement of hemangiomas was most marked, followed by that of HCCs, cholangiocarcinomas, and metastases. The degree of enhancement of HCCs or adenocarcinomas revealed by T1-weighted postcontrast imaging was considerably lower than that of hemangiomas (p < .05). At T2-weighted postcontrast imaging, the signal of hemangiomas decreased slightly.
Figure 5 and Table 3 show CNRs for various tumors at T1-weighted sequences before and after ferumoxides administration. At distributional-phase T1-weighted imaging, hemangiomas exhibited the greatest CNRs relative to surrounding liver, and also the greatest lesion-to-portal vein CNRs (Table 4). A statistically significant difference was noted between the CNRs of hemangiomas and metastases (p < .05) and between those of hemangiomas and HCCs (p < .05).
The accuracy of each reading set is shown in Table 5. Set B (combined distributional phase and accumulation phase imaging) shows that all hemangiomas and cysts were correctly diagnosed. The characterization of focal liver lesions as benign or malignant was better evaluated by set B (overall accuracy=88%) than set A (overall accuracy=75%) (p = .07). When a specific diagnosis was requested, the accuracy of set B decreased to 82%, but was still better than that of set A (p < .05). The additional information provided by ferumoxides-enhanced T1-weighted images obtained during the distributional phase has been shown to be useful for further characterization of focal liver lesions.
The use of ferumoxides in MR imaging of the liver has been the focus of many investigations, and it is now widely accepted that ferumoxides increase the detection rate of focal liver lesions at T2-weighted imaging (4-6). Several authors, however, have encountered difficulties in differentiating benign from malignant lesions at ferumoxides-enhanced MR imaging (11, 19), and until recently, only a small number of reports focused on lesion characterization under this circumstance (8-13). Furthermore, in most previous studies, investigators employed a T2-weighted sequence to characterize focal liver lesions, using the signal loss related to iron particle uptake of Kupffer cells located in benign liver lesions such as focal nodular hyperplasia, adenoma and hemangioma (8-11). However, in a clinical setting, the signal change of focal liver lesions makes it difficult to distinguish metastases from hemangioma on T2-weighted images because metastatic lesions are hyperintense. This can be a more serious problem when small lesions of less than two centimeters are being imaged. Several authors have recently described T1-weighted GRE images obtained 30 minutes to 1 hour after the administration of ferumoxides, noting that they were valuable for characterizing focal liver lesions observed at SPIO-enhanced MR imaging (8-10, 12, 13).
In this study, T1-weighted imaging began within ten minutes of the end of contrast infusion, a rationale based on the results of previous reports regarding the plasma half-life of ferumoxides (19, 20). The most noticeable findings observed in this study were that T1-weighted out-of-phase imaging using minimum echo-time best differentiated hemangiomas from other tumors. As in the findings of previous studies regarding lesion characterization with ferumoxides-enhanced MR imaging (9, 10), hemangiomas showed the highest degree of signal enhancement at T1-weighted imaging. During the distributional phase, only hemangiomas showed a positive mean lesion-to-liver CNR; for other lesions this was negative. Only HCCs showing hyperintensity at precontrast T1-weighted imaging demonstrated hyperintensity at postcontrast T1-weighted out-of-phase imaging using 2.1 ms echo time, but due to the T1 effects of circulating iron particles, the degree of hyperintensity was much lower than that of the portal vein. These results suggest that to improve the efficacy of ferumoxides-enhanced MR imaging for the differentiation of hepatic hemangiomas and malignant liver lesions, T1-weighted GRE imaging using minimum echo-time and performed during the distributional phase, thus demonstrating the T1-effect of circulating iron particles in the blood, should be added to T2-weighted and T2*-weighted imaging.
Quantitative analysis performed in this study showed that the lesion-to-liver CNRs of lesions seen at postcontrast T1-weighted GRE imaging were relatively lower than those observed in the previous studies of Nakayama et al. (12) and Kim et al. (13). This is because during the distributional phase, the contrast uptake of Kupffer cells was small and the liver parenchyma signal therefore decreased only slightly. However, the lesion-to-liver CNRs of lesions seen at T2*-weighted GRE imaging during the accumulation phase of our study were similar to those observed in previous studies (Figs. 3, 4). Given that T1-weighted SPIO-enhanced MR imaging is useful for lesion characterization, not lesion detection, obtaining T1-weighted images during the distributional phase may, because of the presence of circulating iron particles, be better for observing positive enhancement of focal liver lesions.
Owing to the differences demonstrated by the hepatic tumor enhancement profiles, most tumors (82%) could be correctly characterized during the distributional phase of T1- and T2-weighted imaging. Hemangiomas became hyperintense at postcontrast T1-weighted imaging, with a characteristic drop in SI at postcontrast T2-weighted imaging (8, 9). The enhancement pattern of hemangiomas seen at ferumoxides-enhanced imaging is probably due to the high volume of ferumoxide-laden blood present in the enlarged vascular spaces of the lesion. Although HCCs showed some enhancement at ferumoxides-enhanced T1-weighted imaging, the degree of this was significantly lower than for hemangiomas (p < .05). All hypovascular tumors, including metastases (n=12) and cholangiocarcinomas (n=3), showed only slight enhancement (n=4) or peripheral ring enhancement (n=11) at ferumoxides-enhanced T1-weighted imaging, but cysts showed no enhancement. These differences in the characteristics of T1-weighted enhancement, combined with the characteristic drop in SI for hemangiomas at T2-weighted postcontrast imaging, may be of some value in the characterization of these lesions.
It has been suggested that the presence of ring enhancement following the administration of ferumoxides can help characterize focal hepatic lesions (21) and also differentiate benign from malignant liver lesions. In our study, most hypovascular malignant tumors, such as metastases and cholangiocarcinomas (11/15, 73.3%), and a small number of HCCs (3/30, 10%) demonstrated ring enhancement; hemangiomas or cysts, on the other hand, did not. We thus assume that this sign also helps to differentiate hypovascular malignant tumor lesions from other tumors seen at ferumoxides-enhanced T1-weighted imaging.
In ferumoxides-enhanced MR imaging, the fact that the infusion time of the contrast medium is long has given rise to controversy as to whether, for this reason, precontrast imaging is needed. Kim et al. (13) have demonstrated that at T1-weighted minimum-TE GRE imaging, all FNHs and hemangiomas, but no HCCs and only 3% of metastases, were hyperintense, results which suggest that from a clinical standpoint, the need to obtain precontrast images is not great. In our study, however, the signal intensity of HCCs at unenhanced T1-weighted imaging varied: approximately 37% were hyper- or iso-intense compared to the liver at postcontrast T1-weighted out-of-phase GRE imaging. The observed reduction in the signal intensity of liver parenchyma after the infusion of ferumoxides is almost always greater than that of HCCs; a lesion which is hyperintense at precontrast T1-weighted imaging can become more hyperintense and harder to differentiate from hemangioma. Furthermore, although it may save time, the elimination of unenhanced imaging can be detrimental to the characterization of hepatocellular tumors such as FNH, adenoma, and well-differentiated HCC, for which SI relationships with normal unenhanced liver are routinely examined in lesion characterization. For this reason, it would seem prudent to retain the use of unenhanced scanning, though to clarify this issue, further studies may be necessary.
Our study suffers a number of limitations. First, although the study group was large, the relative number of benign lesions was small, thereby reflecting the prevalence of some less common lesions. Second, the presence of some HCCs and some metastases was not pathologically proven. Instead, typical clinical and laboratory findings in combination with typical imaging findings of other imaging modalities were used as the diagnostic criteria. Last, cases of neither focal nodular hyperplasia nor adenoma, which can cause difficulties in differentiating focal liver lesions, were included in this study.
In conclusion, the use of T1-weighted double-echo FLASH imaging, performed soon after the infusion of ferumoxides, improved the differentiation of hepatic hemangiomas from malignant liver lesions and may help increase the accuracy with which malignant lesions are detected.
Figures and Tables
Fig. 1
Liver hemangioma in a 67-year-old woman.
A. Precontrast T1-weighted in-phase gradient-echo image depicts a round hypointense lesion (arrow) in the right lobe of the liver.
B. Precontrast T2-weighted turbo spin-echo image shows a hyperintense lesion (arrow) in the liver.
C. Ferumoxides-enhanced T1-weighted in-phase gradient-echo image obtained during the distributional phase demonstrates positive enhancement of the lesion (arrow), similar to that of the intrahepatic portal vein (open arrow). Note positive enhancement of the abdominal aorta (arrowhead).
D. Ferumoxides-enhanced T2-weighted turbo-spin echo image shows reduced intensity of the lesion (arrow) compared to precontrast image (A).
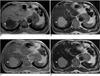
Fig. 2
Liver metastasis in a 67-year-old woman with gastric cancer.
A. Precontrast T2-weighted turbo spin-echo image shows a hyperintense nodule (arrow) in the right lobe of the liver.
B. Postcontrast T1-weighted out-of-phase image demonstrates rim enhancement (arrow) of the peripheral portion of the lesion, which is hypointense to the liver. Note positive enhancement of the intrahepatic portal vein (small arrowheads) and aorta (arrowhead).
C. Postcontrast T1-weighted in-phase image shows that the lesion (arrow) has become hyperintense to the liver.
D. Postcontrast T2*-weighted gradient-echo image obtained during the accumulation phase demonstrates markedly improved lesion (arrow)-to-liver contrast compared to precontrast T2-weighted image.
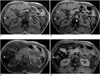
Fig. 3
Surgically-proven cholangiocarcinoma in segment 5 of the liver.
A. Precontrast T2-weighted turbo spin-echo image shows a heterogeneously hyperintense lesion (arrows) with mild capsular retraction in the right lobe of the liver (arrowhead).
B. Precontrast T1-weighted in-phase gradient-echo image depicts a hypointense mass (arrow).
C. On this T1-weighted out-of-phase gradient-echo image obtained after the administration of ferumoxides, the lesion shows peripheral rim enhancement (arrows).
D. T1-weighted in-phase gradient-echo image obtained after the administration of ferumoxides shows that the lesion (arrows) has become slightly hyperintense to the liver.
E. On this T2*-weighted gradient-echo image obtained after the administration of ferumoxides, the lesion (arrow) has become very hyperintense to the liver. Note that this accumulation phase image provides excellent contrast and lesion conspicuity.
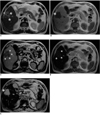
Fig. 4
A 65-year-old man with biopsy-proven hepatocellular carcinoma in the right lobe of the liver.
A. Precontrast T1-weighted out-of-phase gradient-echo image depicts a hyperintense tumor (arrow) with a hypointense capsule in the right lobe of the liver.
B. Postcontrast T1-weighted out-of-phase gradient-echo image obtained during the distributional phase shows that the lesion (arrow) is hyperintense compared to the liver, but much less hyperintense than the branches of the intrahepatic portal vein (open arrows).
C. Postcontrast T1 in-phase gradient-echo image obtained during the distributional phase shows that the lesion (arrow) has become more hyperintense than the liver but is still less hyperintense than the branches of the portal vein (open arrows). Note the presence of multiple hypointense regenerating nodules in the liver parenchyma.
D. Postcontrast T2*-weighted gradient echo image shows markedly improved lesion (arrow)-to-liver contrast due to the substantially decreased liver parenchymal signal.
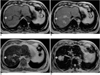
Fig. 5
Lesion-to-liver contrast-to-noise ratio of focal hepatic lesions seen on T1-weighted in-phase and out-of-phase gradient-echo images obtained during the distributional phase

Table 1
Relative Signal Intensity of Focal Liver Lesions in Ferumoxides-Enhanced T1-weighted Dual Echo Sequence

Table 2
Changes in Signal-to-Noise Ratio of Focal Liver Lesions, Liver, and Portal Vein Before and After Administration of Ferumoxides
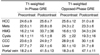
Table 3
Contrast-to-Noise Ratio of Focal Hepatic Lesions at each Ferumoxides-Enhanced MR Imaging Sequence

Note.-HCC=hepatocellular carcinoma, Mets=metastases, HMG=hemangioma, CCC=cholangiocarcinoma, PRE=precontrast, POST=postcontrast, T1W IP=T1-weighted in-phase gradient-echo imaging using 4.2 ms echo time, OP=out-of-phase gradient-echo imaging using 2.1 ms echo time, TSE=turbo spin-echo, GRE=gradient-echo image
Acknowledgments
The authors wish to thank Bonnie Hami, M.A., Department of Radiology, University Hospitals of Cleveland, for her editorial assistance.
References
1. Hagspiel KD, Neidel KFW, Eichenberger AC, Weder W, Marincek B. Detection of liver metastases: comparison of superparamagnetic iron-oxide-enhanced MR imaging at 1.5 T with dynamic CT, intraoperative US, and percutaneous US. Radiology. 1995. 196:471–478.
2. Pena CS, Saini S, Baron BL, et al. Detection of malignant primary hepatic neoplasms with gadobenate dimeglumine (Gd-BOPTA)-enhanced T1-weighted hepatocyte phase MR imaging: results of off-site blinded review in a phase-II multicenter trial. Korean J Radiol. 2001. 2:210–215.
3. Kim SH, Choi D, Lim JH, et al. Optimal pulse sequence for ferumoxides-enhanced MR imaging used in the detection of hepatocellular carcinoma: a comparative study using seven pulse sequences. Korean J Radiol. 2002. 3:87–97.
4. Bluemke DA, Paulson EK, Choti MA, DeSena S, Clavien PA. Detection of hepatic lesions in candidates for surgery: comparison of ferumoxides-enhanced MR imaging and dual-phase helical CT. AJR Am J Roentgenol. 2000. 175:1653–1658.
5. Kanematsu M, Itoh K, Matsuo M, et al. Malignant hepatic tumor detection with ferumoxides-enhanced MR imaging with a 1.5-T system: comparison of four imaging pulse sequences. J Magn Reson Imaging. 2001. 13:249–257.
6. Choi DI, Kim SH, Lim JH, et al. Preoperative detection of hepatocellular carcinoma: ferumoxides-enhanced MR imaging versus combined helical CT during arterial portography and CT hepatic arteriography. AJR Am J Roentgenol. 2001. 176:475–482.
7. Karhunen PJ. Benign hepatic tumors and tumor-like conditions in men. J Clin Pathol. 1986. 39:183–189.
8. Grangier C, Tourniaire J, Mentha G, et al. Enhancement of liver hemangiomas on T1-weighted MR SE images by superparamagnetic iron oxide particles. J Comput Assist Tomogr. 1994. 18:888–896.
9. van Gansbeke D, Metens TM, Matos C, et al. Effects of AMI-25 on liver vessels and tumors on T1-weighted turbo-field-echo images: implications for tumor characterization. J Magn Reson Imaging. 1997. 7:482–489.
10. Mergo PJ, Helmberger T, Nicolas AI, Ros PR. Ring enhancement in ultrasmall superparamagnetic iron oxide MR imaging: a potential new sign for characterization of liver lesions. AJR Am J Roentgenol. 1996. 166:379–384.
11. Parley M, Mergo PJ, Torres GM, Ros PR. Characterization of focal hepatic lesions with ferumoxides-enhanced T2-weighted MR imaging. AJR Am J Roentgenol. 2000. 175:159–163.
12. Nakayama M, Yamashita Y, Mitsuzaki K, et al. Improved tissue characterization of focal liver lesions with ferumoxide-enhanced T1- and T2-weighted MR imaging. J Magn Reson Imaging. 2000. 11:647–654.
13. Kim JH, Kim MJ, Suh SH, Chung JJ, You HS, Lee JT. Characterization of focal hepatic lesions with ferumoxides-enhanced MR imaging: utility of T1-weighted spoiled gradient- recalled echo images using different echo times. J Magn Reson Imaging. 2002. 15:573–583.
14. Oswald P, Clement O, Chambon C, Schouman-Claeys E, Frija G. Liver-positive enhancement after injection of superparamagnetic nanoparticles: respective role of circulating and uptaken particles. Magn Reson Imaging. 1997. 15:1025–1031.
15. Hamm B, Thoeni RF, Gould RG, et al. Focal liver lesions: characterization with nonenhanced and dynamic contrast material-enhanced MR imaging. Radiology. 1994. 190:417–423.
16. Yamashita Y, Hatanaka Y, Yamamoto H, et al. Differential diagnosis of focal liver lesions: role of spin-echo and contrast-enhanced dynamic MR imaging. Radiology. 1994. 193:59–65.
17. Yoshida H, Itai Y, Ohtomo K, et al. Small hepatocellular carcinoma and cavernous hemangioma: differentiation with dynamic FLASH MR imaging with Gd-DTPA. Radiology. 1989. 171:339–342.
18. McFarland EG, Mayo SW, Saini S, et al. Hepatic hemangiomas and malignant tumors: improved differentiation with heavily T2- weighted conventional spin-echo MR imaging. Radiology. 1994. 193:43–47.
19. Oudkerk M, van den Heuvel AG, Wielopolski PA, Schmitz PI, Borel Rinkes IH, Wiggers T. Hepatic lesions: detection with ferumoxides-enhanced T1-weighted MR imaging. Radiology. 1997. 203:449–456.
20. Reimer P, Müller M, Marx C, et al. T1 effects of a bolus-injectable superparamagnetic iron oxide, SH U 555 A: Dependence on field strength and plasma concentration - preliminary clinical experience with dynamic T1-weighted MR imaging. Radiology. 1998. 209:831–836.
21. van Beers B, Gallez B, Pringot J. Contrast-enhanced MR imaging of the liver. Radiology. 1997. 203:297–306.
22. Petersein J, Saini S, Weissleder R. Liver. II: Iron oxide-based reticuloendothelial contrast agents for MR imaging. Clinical review. Magn Reson Imaging Clin N Am. 1996. 4:53–60.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download