Abstract
Thymic epithelial tumor is a distinctive pathologic entity exhibiting variable histologic features and heterogeneous oncologic behavior. Among the various classification systems, that of the World Health Organization has been adopted because of good correlation between histologic appearance and oncologic behavior. Radiologically, a smooth contour and round shape are most suggestive of a type-A tumor, whereas an irregular contour most strongly suggests type C. Pleural seeding is rare in type-A and AB tumors; calcification is suggestive of type B. Type-C tumors are significantly larger and more commonly associated with lymphadenopathy than type B3. At T2-weighted MR imaging, lobular internal architecture is more prominent in types B1, B2, and B3 tumors than in others. However, imaging findings among the various types overlap to some extent, and the ability of imaging studies to differentiate types AB, B1, B2, and B3 is limited.
Thymic epithelial tumors (thymoma and thymic carcinoma), derived from the thymic epithelium, show variable histologic features and heterogeneous oncologic behavior. Various systems of histologic classification of these tumors have been implemented, leading to confusion regarding the nomenclature employed (1). In an effort to establish a universal classification system which reflects the invasiveness and prognosis of thymic epithelial tumors, the World Health Organization (WHO) proposed, in 1999, a consensus classification (2).
Although there is some degree of overlap among the various types of tumor included in the new WHO classification, the CT and MR findings of thymic epithelial tumors help differentiate their various subtypes (3-5). The purpose of this pictorial review is to describe, on the basis of the new WHO classification, the CT, MR and pathologic findings of the various subtypes of thymic epithelial tumors.
The histologic features of thymic epithelial tumors vary, their neoplastic epithelial cells showing differing shapes and degrees of atypia. In addition, various degrees of non-neoplastic lymphoid cells coexist with neoplastic epithelial cells within the tumors (2,6). Numerous classification systems for thymic epithelial tumors have been proposed but have fallen out of favor because the suggested morphologic types lacked consistent prognostic significance. In the mid 1980s, Muller-Hermelink et al. (7) proposed a new functional classification of thymomas based on their histologic and immuno-phenotypic resemblance to cortical or medullary areas of the thymus. The recently published WHO classification of thymomas adopted the Muller-Hermelink classification, but used alpha-numeric terms (3). The new system is based on the morphology of epithelial cells as well as the lymphocyte-to-epithelial cell ratio (Fig. 1): in thymomas are divided into two groups (type A and B) depending on whether the neoplastic epithelial cells and their nuclei are spindle-or oval-shaped (type A), or have a dendritic or epithelioid appearance (type B) (Fig. 2); type AB tumors are those which combine these two morphologies (Fig. 3). Type B tumors are further divided into three subtypes according to the proportional increase in their epithelial component and the emergence of atypical neoplastic cells (Figs. 4-6). All types of thymic carcinomas are classified as type C (Figs. 7, 8). The relationship between the new WHO classification and previous histologic classification systems is summarized in Table 1. The WHO classification reflects the clinical features of these tumors and correlates with prognosis. According to Okumura et al. (8, 9), type-B2 and B3 tumors show aggressive behavior compared with types A, AB, and B1 in terms of invasiveness, postoperative survival, and tumor recurrence. For type-A, AB, or B1 thymomas, there is more likelihood of complete resection than for types B2 or B3.
According to Tomiyama et al. (4), smooth contours and a round shape most strongly suggest a type-A tumor (Table 2) (Fig. 2); type-C tumors are significantly larger than types A and B2 (Figs. 2, 5, 7, 8); irregular contours and mediastinal lymphadenopathy are most suggestive of type C (Figs. 7, 8). Calcification is more frequently seen in type-B1, B2, and B3 tumors than in type AB and type C (Fig. 6). A high degree of homogeneous enhancement tends to indicate type A or AB (Fig. 2); heterogeneous enhancement is seen more often in types B3 and C (Fig. 8). Mediastinal lymphadenopathy is present in 43% of type C tumors, 7% of type AB, but not in other types of thymic epithelial tumors. This feature has, however, been found to be of limited value in differentiating types AB, B1, B2, and B3.
According to Jung et al. (5), CT indicated that atypical (type-B3) thymomas are significantly smaller (mean, 4.7 cm) than thymic carcinomas (type-C thymomas) (mean, 7.2 cm). Invasion of the great vessels, mediastinal lymphadenopathy, extrathymic metastases and phrenic nerve palsy occur only in patients with type-C thymic carcinoma (Figs. 7, 8). The frequencies with which necrosis, intratumoral calcification, pleural effusion, pleural implants, pericardial effusion, and obliteration of the mediastinal fat plane arise are not significantly different between the two types. Do et al. (10) reported that mediastinal lymphadenopathy was present in 40% of thymic carcinomas (type-C thymoma) and 8% of invasive thymomas. With regard to the latter, they did not, however, classify the histopathologic types. Pleural seeding is seen in type-B and C tumors (Figs. 5, 8), but not in type A or AB (5, 10).
At T1-weighted MR imaging, types-A, AB, and B1 thymomas show signal intensity similar to (11) or slightly higher than that of muscle (12-15), while T2-weighted imaging demonstrates signal intensity higher than that of muscle but similar to that of fatty tissue. At Gd-DTPA-enhanced MR imaging, homogeneous and moderate enhancement is observed (Fig. 3). T1-and T2-weighted imaging both demonstrate that the signal intensity of type-B2 and B3 thymomas is the same as that of types A, AB, and B1 (14-17). At T2-weighted imaging, however, most B2 and B3 thymomas manifest inhomogeneous signal intensities with scattered high-intensity areas, shown at pathologic examination to correspond to cystic spaces with or without hemorrhage (14) (Figs. 5, 6). In a study by Sakai et al. (14), T2-weighted imaging indicated that six of 12 invasive thymomas (probably type B2 or B3 according to the WHO classification) had lobular internal architecture, with 1- to 2-mm-thick low-intensity lines (Fig. 6); for the five benign thymomas (probably WHO-type A, AB or B1), this was not, however, the case (Fig. 3). Pathologic examination showed that the lobular internal architecture consisted of round or irregular areas containing tumor cells separated by relatively thick fibrous septa (Fig. 6). Unlike type-A, AB, B1, B2, and B3 tumors, thymic carcinomas (Type C) show relatively low signal intensity at both T1- and T2-weighted MR imaging (15), appearing as slightly inhomogeneous lesions.
The histologic appearance of thymic epithelial tumors, as described in the new WHO classification system, reflects the oncologic behavior of thymomas and thymic carcinomas. An awareness of the various CT and MR findings of the different types of thymic epithelial tumors, as reflected in the WHO histologic classification, may be helpful in clinical practice for the assessment and treatment of patients with thymoma and thymic carcinomas.
Figures and Tables
Fig. 1
Histopathologic demonstration of World Health Organization classification of thymic epithelial tumors (original magnification, ×200; hematoxylin-eosin staining).
A. Type A. The tumor consists of haphazardly distributed spindly epithelial tumor cells (arrows). There are few intermingled lymphocytes (arrowheads).
B. Type AB. The tumor has two lobular components: a discrete type-A lobule (asterisk) and a sharply juxtaposed type-B lobule (star). The former lobule comprises spindly cells with few lymphocytes, while the latter consists of ovoid epithelial cells admixed with numerous lymphocytes. In other cases, these two lobular components can be intricately admixed.
C. Type B1. The tumor consists mostly of a dense population of lymphocytes (mostly immature T cells) (arrows), and some epithelial tumor cells with large nuclei of pale chromatin and small nucleoli (arrowheads). In this tumor, lymphocytes are more abundant than in other thymic epithelial tumors.
D. Type B2. The tumor comprises a of nest of epithelial cells (arrowheads). Lymphocytes (arrows) are less abundant than in type B1.
E. Type B3. The tumor is composed predominantly of polygonal epithelial cells with nuclear atypia (arrows) and some prominent nucleoli (arrowhead). Note that the nuclei of this type are not uniform and bland.
F. Type C. The tumor consists of a nest of squamous cell carcinoma cells (arrows) with severe nuclear atypia and mitotic figures.
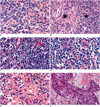
Fig. 2
Type-A thymoma in a 51-year-old woman.
A. Enhanced 7.0-mm-collimation CT scan obtained at the level of the azygos arch demonstrates homogeneous enhancement.
B. Gross pathologic specimen shows that within a pyramid-shaped bilobal thymus, a thymoma (arrow) is present.
C. Photomicrograph (original magnification, × 1; hematoxylin-eosin staining) shows that the tumor is completely surrounded by a fibrous capsule (arrows), which is not infiltrated by tumor cells.

Fig. 3
Type-AB thymoma in a 46-year-old-man.
A. Enhanced 7.0-mm-collimation CT scan obtained at the level of the azygos arch reveals the presence of a round mass in the right anterior mediastinum.
B. T1-weighted (TR/TE, 723/9) MR image obtained at a similar level to A depicts an iso-intense mass.
C. Gd-enhanced T1-weighted MR image demonstrates strong tumoral enhancement.
D. T2-weighted (TR/TE, 5700/89) MR image shows a mass lesion with slightly higher signal intensity than muscle.
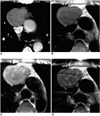
Fig. 4
Type-B1 thymoma in a 48-year-old-man.
A. Enhanced 10-mm-collimation CT scan obtained at the level of the aortic arch depicts a well-enhanced homogeneous lobulated mass in the left anterior mediastinum.
B. Gross pathologic specimen reveals the presence of well-formed lobules separated by dense fibrous septa (arrows).
C. Photomicrograph (original magnification, × 1; hematoxylin-eosin staining) depicts lobulated internal architecture separated by dense fibrous septa (arrows).
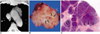
Fig. 5
Type-B2 thymoma in a 35-year-old woman.
A. Enhanced 10-mm-collimation CT scan obtained at the level of the right interlobar pulmonary artery shows that in the anterior mediastinum, a slightly attenuated heterogeneous mass is present.
B. CT scan (10-mm collimation) obtained at the level of the suprahepatic inferior vena cava depicts a pleural mass lesion (arrows) with low attenuation.
C. T1-weighted MR image obtained at a similar level to A shows a mass lesion with high-signal intensity.
D. T2-weighted image depicts a slightly heterogeneous high-signal lesion.
E. Photomicrograph (original magnification, × 1; hematoxylin-eosin staining) shows an invasive tumor bud (arrowheads) penetrating the capsule where there is no fibrous sleeve.
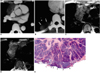
Fig. 6
Type-B3 thymoma in a 50-year-old man with chest pain.
A, B. Enhanced 7.0-mm-collimation CT scans obtained at the levels of the aortic arch (A) and main bronchi (B) depict a partly lobulated and irregularly-marginated mass in the anterior mediastinum. There is invasion of mediastinal fat and adjacent lung. Note the presence of nodular calcification (arrow) within the mass lesion depicted in A.
C. T1-weighted MR image obtained at the level of the aortic arch reveals the presence of a mass lesion with slightly high signal intensity.
D. T2-weighted MR image depicts a lesion of higher signal intensity, broadly attached to the aorta. Note the lobulated internal architecture, separated by low-signal intensity septa (arrows).
E. Gross specimen shows pericardial, pleural, aortic, and lung parenchymal involvement (arrowheads). At the cut surface, partially lobulated internal architecture is noted, with thick fibrous septa (arrows).
F. Photomicrograph (original magnification, × 1; hematoxylin-eosin staining) discloses no intact capsule (arrows), with lobules of polygonal epithelial cells showing mild nuclear atypia.
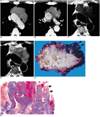
Fig. 7
Type-C thymic carcinoma in a 30-year-old woman with chest pain.
A, B. Enhanced 7.0-mm-collimation CT scans obtained at the levels of the aortic arch (A) and main bronchi (B) show a heterogeneously enhancing mass with irregular contour. Also note that the tumor has invaded mediastinal fat (arrows) and the left upper lobe.
C. Gross pathologic specimen demonstrates the invasion of mediastinal fal (arrows) by an irregular mass.
D. Photomicrograph (original magnification, × 1; hematoxylin-eosin staining) depicts a mass with jigsaw puzzle-like lobules separated by dense fibrous septa. It proved to be type-C thymic carcinoma, with keratinizing squamous cell carcinoma as the main component.
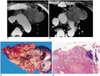
Fig. 8
Type-C thymic carcinoma in a 53-year-old man with chest pain.
A. Enhanced 7.0-mm-collimation CT scan obtained at the level of the left innominate vein shows that the anterior mediastinum contains a heterogeneously enhancing irregular mass. Also note nodular pleural thickening (arrowheads) in both hemithoraces, representing pleural seeding.
B. Lung window image obtained at the level of the bronchus intermedius demonstrates the presence of small nodules (arrowheads) along major and minor fissures. Also note the presence of pulmonary nodules in the right upper lobe (arrows), representing hematogeneous metastasis.

References
1. Shimosato Y, Mukai K. Tumors of the mediastinum. Atlas of tumor pathology. 1997. Washington, DC: Armed Forces Institute of Pathology;3rd series, fascicle 21.
2. Rosai J, Sobin LH. Histological typing of tumors of the thymus. International histological classification of tumours. 1999. 2nd ed. New York: Springer.
3. Tomiyama N, Müller NL, Ellis SJ, et al. Invasive and non-invasive thymoma: distinctive CT features. J Comput Assist Tomogr. 2001. 25:388–393.
4. Tomiyama N, Johkoh T, Mihara N, et al. Using the World Health Organization classification of thymic epithelial neoplasms to describe CT findings. AJR Am J Roentgenol. 2002. 179:881–886.
5. Jung KJ, Lee KS, Han J, Kim J, Kim TS, Kim EA. Malignant thymic epithelial tumors: CT-pathologic correlation. AJR Am J Roentgenol. 2001. 176:433–439.
6. Suster S, Moran CA. Thymoma, atypical thymoma, and thymic carcinoma: A novel conceptual approach to the classification of thymic epithelial neoplasms. Am J Clin Pathol. 1999. 111:826–833.
7. Müller-Hermelink HK, Marino M, Palestro G. Pathology of thymic epithelial tumors. Curr Top Pathol. 1986. 75:207–268.
8. Okumura M, Miyoshi S, Fujii Y, et al. Clinical and functional significance of WHO classification of human thymic epithelial neoplasms. A study of 146 consecutive tumors. Am J Surg Pathol. 2001. 25:103–110.
9. Okumura M, Ohta M, Tateyama H, et al. The World Health Organization histologic classification system reflects the oncological behavior of thymoma: a clinical study of 273 patients. Cancer. 2002. 94:624–632.
10. Do YS, Im J-G, Lee BH, et al. CT findings in malignant tumors of thymic epithelium. J Comput Assist Tomogr. 1995. 19:192–197.
11. Herold CJ, Zerhouni EA. Higgins CB, Hricak H, Helms CA, editors. The mediastinum and lungs. Magnetic resonance imaging of the body. 1992. 2nd ed. New York: Raven Press.
12. Molina PL, Siegel MJ, Glazer HS. Thymic masses on MR imaging. AJR Am J Roentgenol. 1990. 155:495–500.
13. Ikezoe J, Takeuchi N, Johkoh T, et al. MRI of anterior mediastinal tumors. Radiat Med. 1992. 10:176–183.
14. Sakai F, Sone S, Kiyono K, et al. MR imaging of thymoma: radiologic-pathologic correlation. AJR Am J Roentgenol. 1992. 158:751–756.
15. Kushihashi T, Fujisawa H, Munechika H. Magnetic resonance imaging of thymic epithelial tumors. Crit Rev Diagn Imaging. 1996. 37:191–259.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download