Abstract
Objective
To determine the feasibility of transcaval transjugular intrahepatic portosystemic shunt (TIPS) creation in patients with unusual anatomy between the hepatic veins and portal bifurcation, and inaccessible or inadequate hepatic veins.
Materials and Methods
Transcaval TIPS, performed in six patients, was indicated by active variceal bleeding (n=2), recurrent variceal bleeding (n=2), intractable ascites (n=1), and as a bridge to liver transplantation (n=1). The main reasons for transcaval rather than classic TIPS were the presence of an unusually acute angle between the hepatic veins and the level of the portal bifurcation (n=3), hepatic venous occlusion (n=2), and inadequate small hepatic veins (n=1).
Results
Technical and functional success was achieved in all patients. The entry site into liver parenchyma from the inferior vena cava was within 2 cm of the atriocaval junction. Procedure-related complications included the death of one patient due to hemoperitoneum despite the absence of contrast media spillage at tractography, and another suffered reversible hepatic encephalopathy.
Transjugular intrahepatic portosystemic shunt (TIPS) has been successfully used to manage uncontrolled variceal bleeding, intractable abdominal ascites, hepatic hydrothorax, and Budd-Chiari syndrome (1-6). Classically, the shunt tract is created between the hepatic and portal vein, but when the hepatic veins are inaccessible or inadequate because of occlusion, as in Budd-Chiari syndrome or tumor, and when the portal vein cannot be punctured because of the cephalad location of its bifurcation relative to the hepatic veins, the transcaval route is inevitable. Previously reported cases of transcaval TIPS involved localization of the right portal vein followed by the percutaneous portal vein approach (7-9), though one recent study (10) reported blind puncture of the portal vein from the IVC in five cases. In this study, transcaval TIPS were created in the classic manner, using only the right internal jugular vein approach and blind transcaval puncture of the right portal vein, without additional percutaneous localization of the right portal vein. The purpose of this study was to evaluate the feasibility of transcaval TIPS creation in patients with unusual anatomy between the hepatic veins and portal bifurcation, and inadequate or inaccessible hepatic veins.
Between May 1999 and April 2001, transcaval TIPS was performed in six patients (four men and two women; age range, 30-73 years). The clinical data are listed in Table 1. Indications for TIPS were active variceal bleeding (n=2), recurrent variceal bleeding (n=2), intractable ascites (n=1), and as a bridge to liver transplantation (n=1). All patients had liver cirrhosis, and three had hepatocellular carcinoma with underlying viral liver cirrhosis. In two, Budd-Chiari syndrome was associated; this was subsequent to Behcet's disease in one patient and idiopathic in the other. The main reasons for transcaval rather than classic TIPS were the presence of an unusually acute angle between the hepatic veins and the level of the portal bifurcation (n=3), hepatic venous occlusion (n=2), and inadequate small hepatic veins (n=1). The Institutional Review Board approved the procedure, and informed consent was obtained from each patient or their legal guardian.
In two patients with hepatic venous occlusion and one with inadequate small hepatic veins, transcaval TIPS was first attempted, and in three with patent and adequate hepatic veins, the treatment of first choice was classical TIPS. After these techniques failed due to the unusually acute angle between the hepatic veins and the level of the portal bifurcation, venacavography of the inferior vena cava (IVC) was performed to confirm the presence of its retrohepatic segment, and direct cavoportal puncture was attempted. A 16-gauge Colapinto needle and a 9-F Teflon sheath (Transjugular Liver Access Set; Cook, Bloomington, Ind., U.S.A.) were coaxially loaded over a guide-wire through the right internal jugular vein. Percutaneously placed anatomic landmarks were not used. After CT scanning, the relationship between the retrohepatic segment of the IVC and the portal vein was reconstructed in the operator's mind, and the ventral IVC wall was punctured and the needle directed toward the portal vein. If necessary, the Colapinto needle curve was made more acute to achieve the required posterior-to-anterior trajectory through the liver. After portal vein puncture, a 0.035-inch hydrophilic guide-wire (Terumo, Tokyo, Japan) was introduced through the needle and placed in the splenic or superior mesenteric vein, and direct portography was performed using a 5-F angiographic catheter. After measuring the pressure in the main portal vein, the needle tract was dilated with an 8- or 10-mm balloon catheter (Medi-Tech/Boston Scientific, Watertown, Mass., U.S.A.). A 9-F sheath was placed in the tract, and direct tractography was performed to visualize the extravasation of contrast medium and biliary communication. After verifying that these were absent, a self-expandable Wallstent (10 mm in diameter / 7 or 9 cm in length) was deployed. Post-stenting balloon dilation with an 8- or 10-mm balloon was performed to achieve a portosystemic pressure gradient of less than 15 mmHg, as measured from the main portal vein to the right atrium.
The TIPS tract was evaluated by color and spectral Doppler ultrasonography. The first control study was performed 1-5 days after the initial procedure, and subsequent examination was followed by control studies at three-month intervals. Indirect portography was available in patients with hepatocellular carcinoma.
The results of transcaval TIPS creation are listed in Table 1. Technical and functional success was achieved in all patients (Figs. 1-3).
The entry site into liver parenchyma from the IVC was within 2 cm of the atriocaval junction. The puncture site involved was the right portal vein in four patients, the left portal vein in one, and the bifurcation level of the right and left portal vein in one. Before TIPS creation, the mean pressure gradient between the main portal vein and right atrium was 33 mmHg, and TIPS creation caused this to decrease significantly, to 12 mmHg. In addition, post-TIPS direct portography demonstrated rapid flow into the shunt tract.
Procedure-related complications included the death of one patient due to hemoperitoneum despite the absence of contrast media spillage at tractography, and another suffered reversible hepatic encephalopathy.
Because this first patient, who had intractable ascites, died one day after the procedure (case 3), and another underwent liver transplantation one month after the procedure (case 4), the results of follow-up were available in only four of the six patients. The symptoms of variceal bleeding were well controlled. Two of the four patients (cases 2 and 5) who were followed up showed primary patency for more than sixteen months. TIPS was revised in two patients (cases 1 and 6), and in both, gastric fundal variceal bleeding recurred. Direct portography performed via the right internal jugular vein approach demonstrated malfunction of the stent due to diffuse intimal hyperplasia. In case 1, the TIPS tract was dilated with a 10-mm balloon, and the portosystemic pressure gradient decreased from 25 to 10 mmHg. Despite this drop to an effective range, a markedly dilated gastric fundal varix persisted, and further variceal embolization was performed by injecting histoacryl. In case 6, dilatation with a 10-mm balloon alone did not lower the portosystemic pressure gradient to the effective range, so a 10-mm/9-cm Wallstent was again deployed, followed by post-stenting dilatation with a 10-mm balloon. The portosystemic pressure gradient then decreased from 25 to 8 mmHg, and variceal filling was not demonstrated. Secondary patency was well maintained during the follow-up period.
Transcaval TIPS has been documented in various reports of different conditions. It was performed, for example, in a patient with acute Budd-Chiari syndrome and a thrombosed surgical mesocaval shunt and stenosis of the IVC (7). Another case involved a patient who lacked the hepatic veins capable of supporting TIPS (8), and other reports have described a case in which transfemoral transcaval TIPS was performed to relieve occlusion of both internal jugular veins (9), and unsuccessful revision of previously performed TIPS (10).
If a transcaval shunt is to be safe, it must exit the IVC at a site that is well invested by the fibrous tissue of the retroperitoneum. If the shunt exits a caudal, intraperitoneal portion of the IVC, then bleeding through the porous stent mesh at this site might occur. The retrohepatic IVC, or safe zone, has been measured in two cadaver studies. Chang et al. (11) reported that in 60 livers taken from adult cadavers and free from gross pathologic change, the average length of the retrohepatic segment was 7.1 cm, while Camargo et al. (12) found that in 30 pathologically sound livers from a similar source, the average length of this segment was 6.7 (range, 3.5-10.9) cm. Saxon and Keller (13) stated that the hepatic vein usually enters the IVC within 2 cm of the right atrium, and that a point not more than 6 cm from this was therefore the safest puncture point within the ventral wall of the IVC. They also argued that since the caval exit site is posterior to the standard exit site through the hepatic vein, excursion of the curved puncture needle should be more ventral.
Because the length of the retrohepatic segment of the IVC may vary in patients with cirrhosis, two important points should be borne in mind when performing transcaval TIPS. First, the puncture site should be adjacent to the native orifice of the hepatic veins. The advantages of this are that puncture of the retrohepatic segment of the IVC is thus guaranteed, and during puncture, the Colapinto needle will not be pushed back. Second, it is crucial to ensure that no leakage occurs at either the caval entry site or the tract, and that there is no communication between the biliary tree and the tract. Digital subtraction angiography after the injection of contrast medium through a sidestream adaptor and with a guide-wire appropriately positioned is able to demonstrate the presence or absence of contrast medium leakage into the extrahepatic space and communication between the biliary tree and the tract.
Where there is biliary communication, bile leakage into the shunt tract can induce thrombosis and pseudointimal hyperplasia, and the use of a stent-graft to prevent early tract occlusion is recommended. In addition, where there is extravasation of contrast medium, the location and amount of this, and whether it is contained extravasation or free spillage, should first be evaluated. Possible therapeutic options, including occlusion, bare stent insertion, and stentgraft insertion, should then be considered. Seong et al. (10) reported that in three of five cases in which transcaval TIPS involved the extravasation of contrast medium, this was confined to hepatic subcapsular space or the pericaval and subdiaphragmatic area. They stated that after tract dilatation and bare stent placement in these patients, there was no evidence of bleeding while their condition was stable, and the reason this did not occur was that the shunt tract exited the retrohepatic segment of the IVC at a site well invested by retroperitoneal fibrous. However, if contrast medium is extravasated as a result of free spillage, particularly through leakage from the extrahepatic portion of the portal vein, treatment options include 1) portal vein tamponade with an occlusion balloon, and rapid shunt formation through a different tract; 2) placement of a stent graft; or 3) completion of the intra-tract procedure by placement of a conventional bare mesh stent (14-16). A few recent reports have stated that stent-graft deployment in a TIPS tract can increase the primary patency period, and that the use of a stent-graft of appropriate size may therefore be a feasible therapeutic option (17, 18).
In patients with a large amount of ascites and small liver volume, the risk of hemoperitoneum during repeated puncture is high, due to laceration of the inferior liver capsule. An example of this is case 3: although contrast medium had not leaked into the extrahepatic space, the patient died due to hemoperitoneum, an outcome which may be due to laceration of the inferior liver capsule due to repeated puncture.
One of the suggested advantages of transcaval TIPS is that in theory, transcaval shunts may provide longer patency because the hepatic vein is entirely avoided. However, until a large series is reported, the superiority of the procedure in this respect remains unproven.
It has been reported that transcaval TIPS in patients awaiting orthotopic liver transplant offers a further benefit by positioning the outflow (caval) end of the stent in the intrahepatic IVC segment (9, 19, 20). This can avoid both supra- and infrahepatic caval cuffs and can subsequently decrease any theoretical risk of interference with inferior vena cava segments crucial for orthotopic liver transplant (9).
Although the number of patients in this study was limited, the favorable results indicate that transcaval TIPS creation is feasible in patients with unusual anatomy between the hepatic veins and the portal bifurcation, and inaccessible or inadequate hepatic veins.
Figures and Tables
Fig. 1
Case 2. A 41-year-old man with active variceal bleeding. A, B. Sequential MR images (1 cm slice thickness) depict approximation of the right proximal hepatic vein (arrow in A) and right portal vein (arrow in B).
C. After classic puncture at the proximal hepatic vein, only the peripheral portal branch was punctured. Approximation of the estimated proximal right hepatic vein and right portal vein is noted (arrows).
D. Following transcaval TIPS creation using a Wallstent 10 mm in dianeter and 7 cm in length, the portosystemic gradient decreased from 34 mmHg to 13 mmHg. Subsequent direct portography demonstrated good flow through the stent and decreased flow into the coronary varix.
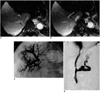
Fig. 2
Case 4. A 38-year-old woman who was a candidate for liver transplantation.
A. Contrast-enhanced CT scan shows a thrombosed hepatic vein (arrow), inhomogeneous parenchymal enhancement, and a substantial amount of ascites, compatible with Budd-Chiari syndrome.
B, C. IVC venogram (B) and angiogram (C) obtained after direct intrahepatic puncture show the collateralization typical of Budd-Chiari syndrome.
D. Transcaval TIPS was performed due to occlusion of hepatic veins.
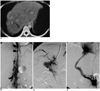
Fig. 3
Case 6. A 73-year-old man with active variceal bleeding.
A. CT scan shows occlusion of the right portal vein due to repeated transcatheter arterial chemoembolization and gastric fundal varices.
B, C. Because the middle (B) and left (C) hepatic veins were too small, classic TIPS was not possible.
D. Transcaval TIPS was performed via the left portal vein.
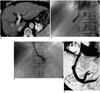
Table 1
Clinical Data and Results of Transcaval Transjugular Intrahepatic Portosystemic Shunt

Note.-TIPS=transjugular intrahepatic portosystemic shunt, PrePG=pre-TIPS pressure gradient (mmHg), PostPG=post-TIPS pressure gradient (mmHg), LC=liver cirrhosis, HCC=hepatocellular carcinoma, BD=Behcet's disease, BCS=Budd-Chiari syndrome, HV=hepatic vein, PV=portal vein, E=encephalopathy, N/A=not applicable, *the patient expired one day after the procedure, and thus the patency rate could not be estimated, †the patient underwent liver transplantation one month after the procedure, and the patency rate could not, therefore, be estimated.
References
1. Richter GM, Noeldge G, Palmaz JC, et al. Transjugular intrahepatic portocaval stent shunt: preliminary clinical results. Radiology. 1990. 174:1027–1030.
2. Zemel G, Katzen BT, Becker GJ, Benenati JF, Sallee DS. Percutaneous transjugular portosystemic shunt. JAMA. 1991. 266:390–393.
3. Rossle M, Haag K, Ochs A, et al. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N Engl J Med. 1994. 330:165–171.
4. Ochs A, Rossle M, Haag K, et al. The transjugular intrahepatic portosystemic stent-shunt procedure for refractory ascites. N Engl J Med. 1995. 332:1192–1197.
5. Blum U, Rossle M, Haag K, et al. Budd-Chiari syndrome: technical, hemodynamic, and clinical results of treatment with transjugular intrahepatic portosystemic shunt. Radiology. 1995. 197:805–811.
6. Kamath PS, McKusick MA. Transvenous intrahepatic portosystemic shunts. Gastroenterology. 1996. 111:1700–1705.
7. Rogopoulos A, Gavelli A, Sakai H, McNamara M, Huguet C. Transjugular intrahepatic portosystemic shunt for Budd-Chiari syndrome after failure of surgical shunting. Arch Surg. 1995. 130:227–228.
8. Haskal ZJ, Duszak R Jr, Furth EE. Transjugular intrahepatic transcaval portosystemic shunt: the gun-sight approach. J Vasc Interv Radiol. 1996. 7:139–142.
9. Soares GM, Murphy TP. Transcaval TIPS: indications and anatomic considerations. J Vasc Interv Radiol. 1999. 10:1233–1238.
10. Seong CK, Kim YJ, Shin TB, Park HY, Kim TH, Kang DS. Transcaval TIPS in patients with failed revision of occluded previous TIPS. Korean J Radiol. 2001. 2:204–209.
11. Chang RW, Shan-Quan S, Yen WW. An applied anatomical study of the ostia venae hepaticae and the retrohepatic segment of the inferior vena cava. J Anat. 1989. 164:41–47.
12. Camargo AM, Teixeira GG, Ortale JR. Anatomy of the ostia venae hepaticae and the retrohepatic segment of the inferior vena cava. J Anat. 1996. 188:59–64.
13. Saxon RR, Keller FS. Technical aspects of accessing the portal vein during the TIPS procedure. J Vasc Interv Radiol. 1997. 8:733–744.
14. Haskal ZJ, Cope C, Soulen MC, Shlansky-Goldberg RD, Baum RA, Redd DC. Intentional reversible thrombosis of transjugular intrahepatic portosystemic shunts. Radiology. 1995. 195:485–488.
15. Brountzos EN, Alexopoulou E, Koskinas I, Thanos L, Papathanasiou MA, Kelekis DA. Intraperitoneal portal vein bleeding during transjugular intrahepatic portosystemic shunt: treatment with stent-graft placement. AJR Am J Roentgenol. 2000. 174:132–134.
16. Davis AG, Haskal ZJ. Extrahepatic portal vein puncture and intra-abdominal hemorrhage during transjugular intrahepatic portosystemic shunt creation. J Vasc Interv Radiol. 1996. 7:863–866.
17. Haskal ZJ, Davis A, McAllister A, Furth EE. PTFE-encapsulated endovascular stent-graft for transjugular intrahepatic portosystemic shunts: experimental evaluation. Radiology. 1997. 205:682–688.
18. Haskal ZJ. Improved patency of transjugular intrahepatic portosystemic shunts in humans: creation and revision with PTFE stent-grafts. Radiology. 1999. 213:759–766.
19. Woodle ES, Darcy M, White HM, et al. Intrahepatic portosystemic vascular stents: a bridge to hepatic transplantation. Surgery. 1993. 113:344–351.
20. Lerut JP, Laterre PF, Goffette P, et al. Transjugular intrahepatic portosystemic shunt and liver transplantation. Transpl Int. 1996. 9:370–375.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download