Abstract
Sclerosing stromal tumor (SST) of the ovary is a very rare sex cord stromal tumor occurring in a younger age group than other types of stromal tumors and most commonly accompanied by menstrual irregularity. Several unique histologic features including pseudolobulation, sclerosis and prominent vascularity are clearly reflected at ultrasonography and MRI. We report the ultrasonographic and MR features of three cases of histologically confirmed SSTs, and relate them to the pathological findings.
Sclerosing stromal tumor (SST) of the ovary is a rare benign tumor that is classified as a sex cord stromal tumor (1-5). SSTs show unique clinical and pathologic features that distinguish them from other ovarian stromal tumors, and since 1999, several reports describing their ultrasonographic and MR findings have been published (1, 2, 4, 5). We report the ultrasonographic and MR features of three cases of histologically confirmed SSTs and relate them to the pathologic findings.
A 16-year-old girl presented with a history of menstrual irregularity extending over several months. Physical examination revealed a round palpable mass in the lower abdomen, though routine laboratory findings, including tumor markers, were normal. Ultrasonography disclosed a large round mass, mainly solid and with small central hypoechoic and anechoic areas, in the left adnexal region. Power Doppler examination revealed the presence of multiple tortuous vessels, extending from the periphery to the central portion, and with a "spoke-wheel" appearance (Fig. 1A).
In order to further characterize the mass, MR imaging was performed, and this revealed that its central portion had the same signal intensity as water and was wider than the cystic portion seen at ultrasonography; homogeneous low signal intensity was apparent at T1-weighted imaging (Fig. 1B), and high signal intensity at T2-weighted imaging (Fig. 1C). At both weightings, the peripheral portion of the mass was slightly hyperintense relative to muscle; T2-weighted imaging depicted a thin, low-intensity rim surrounding the mass. Dynamic contrast-enhanced MRI demonstrated early strong enhancement of the peripheral portion of the mass, a finding similar to that of the vessels (Fig. 1D). Delayed imaging demonstrated progressive centripetal and prolonged enhancement (Fig. 1E), though the central portion was entirely unenhanced. A small amount of ascites surrounded the mass (Fig. 1C).
Laparotomy depicted a 5×6×6 cm-sized soft solid mass in the left ovary. Left salpingo-oophorectomy with partial omentectomy was performed, and about 60 cc of serous ascitic fluid was present. The cut surface was lobulated, yellowish white in color, and there were central areas of edema (Fig. 1F). Microscopic examination revealed a pseudolobular pattern in which cellular nodules were separated by areas of densely collagenous or edematous connective tissue which contained fewer cells. The nodules were an admixture of fibroblasts and rounded vacuolated cells, with prominent thin-walled vessels (Fig. 1G). Areas of strong enhancement correlated with the cellular areas in which the vascular network was prominent, and non-enhancing areas were composed of collagenous and edematous connective tissue. The ovarian capsule was intact and represented as a hypointense rim at MR imaging, and the ascitic fluid contained no tumor cells. Immunohistochemical staining was positive for vimentin, smooth muscle actin, and progesterone receptor.
A 26-year-old woman complained of vaginal bleeding and menorrhagia, which had lasted for six days. A large mass was palpable in the lower abdomen, though a urine pregnancy test was negative and routine laboratory findings were unremarkable. Serum levels of CA 19-9, alpha-fetoprotein and carcinoembryonic antigen were all within normal limits. Ultrasonography revealed slight ascites and a solid mass in the left adnexal region.
At MR imaging, the mass showed findings similar to those of case 1. At both T1- and T2-weighted imaging, a thin low-intensity rim was visible, and at the periphery, signal intensity was slightly higher than that of muscle. The central zone of the mass was hypointense at T1-weighted imaging and bright at T2-weighted imaging (Figs. 2A, B). After IV administration of gadolinium-DTPA, peripheral nodular enhancement was demonstrated (Fig. 2C); in this case, dynamic images were not obtained. A cul-de-sac contained a small amount of ascites, but neither lymphadenopathy nor peritoneal tumor seeding was apparent.
At surgery, the mass measured 6×5×5 cm; immunohistochemical staining was positive for smooth muscle actin and focally positive for vimentin. The final pathologic diagnosis was sclerosing stromal tumor.
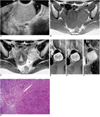
A 39-year-old-woman presented with a history of irregular menstrual cycles and mild lower abdominal pain. A large mass was palpable in the left lower quadrant. Transvaginal ultrasonography revealed the presence of a large mainly solid mass in the left adnexal region (Fig. 3A), and at the periphery, three oval-shaped anechoic cystic lesions were visible. Color Doppler examination demonstrated multiple vascular channels, predominantly peripherally. A urine pregnancy test was negative, and a routine blood test was normal. There was no evidence of malignant tumors at other sites.
T1-weighted MR imaging of the mass demonstrated homogeneous signal intensity, slightly higher than that of muscle (Fig. 3B). At T2-weighted imaging, the mass was heterogeneously hyperintense (Fig. 3C), and both T1- and T2-weighted images revealed signal-void tubular structures around it. Dynamic contrast-enhanced MR imaging demonstrated marked early nodular enhancement of the peripheral portion of the mass, which was as high as that of the vessels (Fig. 3D). Delayed imaging revealed progressive centripetal enhancement, except in small scattered areas of the cyst and cleft (Figs. 3E, F). Three oval-shaped cystic lesions and hyperintense band-like areas surrounded the mass, and in the pelvis there was slight ascites.
At surgery, the left ovary was found to have been replaced by the mass, which measured 5.5×4.5×4 cm. Its gross appearance was that of a diffusely homogeneous white, sclerotic, solid lesion with multiple foci of yellow spots. Microscopic examination revealed a pseudolobular growth pattern, with mixed cellular and hypocellular areas (Fig. 3G). The former correlated with areas of early strong enhancement at dynamic MR imaging, and the latter with areas of prolonged enhancement, and multiple vascular structures were observed. Peripheral cysts proved to be follicular, and hyperintense band-like areas surrounding the mass corresponded to edematous ovarian cortex. The ascites contained no tumor cells.
Ovarian sex cord stromal tumors are classified, inter alia, as granulosa stromal cell tumors, Sertoli's stromal tumors, and steroid cell tumors. SSTs, along with granulosa cell tumors, thecomas and fibromas, are granulosa stromal cell tumors (6), and were first described as a distinct entity among ovarian sex cord stromal tumors by Chalvardjian and Scully in 1973 (7). They are known to have the following characteristic clinical features: first, they usually occur in a younger age group, among those aged 27 or 28 years (1, 4, 5); whereas other types of stromal tumors are most common in the fifth and sixth decades, about 80% of SSTs are encountered during the second and third decades (1-3, 5, 8). In our study, two of three patients were aged less than 30, and the other was 39. Second, the most common presenting symptom is menstrual irregularity (3-5), and this occurred in all our three patients. Third, ascites occurs, but is rare (2, 4, 7, 9). In all our three cases, however, there was some ascites around the mass or in the cul-de-sac, and in several previous case reports the presence of ascites was also noted (1-2). To date, malignant SST has not been reported (1, 4). In our study, all three SSTs originated from the left ovary, and in several previously published reports, occurrence on this same side was also described (1-3, 5). In another study, however, this was not the case (4).
Hormonal activity has been reported in a few documented cases (1, 9-10), and though none of our patients underwent hormonal assay, they demonstrated no symptoms suggesting hormonal activity.
In a previous report by Lee et al. (4), SSTs were shown at ultrasonography to be solid and cystic adnexal masses with centrally located, multiple, round or cleft-like cysts, and two patients in our study, in one of whom the cyst was entirely solid and homogeneously echogenic, showed similar features. Among the cases described by Lee et al., ascites was uncommon, occurring in only two of the seven. Ascites was, however, demonstrated at ultrasonography in all our patients. In their report, color Doppler ultrasonography of SSTs revealed prominent vascularity in the peripheral portion and central intercystic spaces; power Doppler ultrasonography was not performed. In one of our patients, however, this modality showed a peculiar finding: the so-called "spoke-wheel appearance" , i.e., peripherally located arc-shaped large vessels and multiple vertically oriented centripetal vascular flow. We believe that because of its higher sensitivity to flow signals, power Doppler ultrasonography permits continuous delineation of vascular flow.
The T2-weighted MRI findings of SSTs are a round or oval-shaped mass with hyperintense cystic components, and a heterogeneous solid component of intermediate to high signal intensity (1, 2, 5, 8). At dynamic contrast-enhanced imaging, early peripheral enhancement with centripetal progression has been reported (2, 5, 8).
Microscopically, SSTs are characterized by a number of distinctive features: 1) a pseudolobular growth pattern, in which cellular areas are separated by edematous and collagenous hypocellular areas; 2) collagenous sclerosis within the cellular areas; 3) marked vascularity, with a "hemangiopericytomatous" pattern; and 4) heterogeneity of the cell population (1, 2). Except for this last-mentioned, our MR findings clearly reflected these pathologic features. At T2-weighted imaging, pseudolobulation was represented by an admixture of hypointense lesions set against a background of hyperintense stroma; pronounced sclerosis within the cellular nodules correlated with the hypointense areas. Prominent vascularity within cellular areas explained the marked and early tumoral enhancement we observed. Pathologic specimens obtained from our patients showed that the cystic areas were small; in fact the areas of high signal intensity seen at T2-weighted imaging and the unenhanced areas observed at dynamic imaging correlated with areas of hypocellularity and edema, and the thick or thin peripheral rim corresponded to the compressed ovarian cortex and capsule. In an earlier report, Ihara et al. (1) concluded that the presence of a thick peripheral rim consisting of compressed ovarian stroma can help differentiate SSTs from other stromal tumors. SSTs, they claimed, have a thick peripheral rim for two reasons: first, they occur in younger women with large ovaries; second, the tumors are slow growing. Fibromas and thecomas, on the other hand, are common in older women with atrophied ovarian stroma that are hardly visible, even if present at a tumor's periphery. However, our report showed that the peripheral rim of SSTs varies in thickness, and may be very thin. In one of our patients (case 3), the peripheral ovarian cortex contained three functional cysts.
The differential diagnosis of SSTs should include thecoma/fibroma, metastases, and malignant epithelial ovarian tumors. Fibromas/thecomas usually show low signal intensities at T2-weighted imaging, and slow and prolonged enhancement at dynamic MRI. Ovarian metastases and malignant epithelial tumors usually occur in older patients, and dynamic MR imaging does not usually reveal progressive centripetal enhancement.
In summary, SSTs are mixed cystic and solid tumors with central round or cleft-like cysts and show quite specific imaging findings, especially at power Doppler ultrasonography and dynamic MRI. Findings of large peripherally located vessels and centripetal vascular flow ("spoke-wheel appearance") at power Doppler ultrasonography, and early peripheral enhancement with centripetal progression at dynamic MRI, are usual. Acknowledgment of these specific findings of SSTs permits the accurate preoperative diagnosis of these benign tumors and promotes less invasive surgery (such as laparoscopic tumor excision) rather than oophorectomy.
Figures and Tables
Fig. 1
Sclerosing stromal tumor of the left ovary in a 16-year-old girl.
A. Peripheral arc-like vessels with a vertically oriented centripetal vascular network (the so-called "spoke-wheel appearance") in an oval-shaped left adnexal mass are clearly demonstrated at power Doppler ultrasonography.
B. Axial T1-weighted MR image depicts a left adnexal mass (arrows). The peripheral portion of the mass is slightly hyperintense relative to muscle, and the central portion is hypointense.
C. Axial T2-weighted MR image shows a slightly hyperintense peripheral portion relative to muscle, and a hyperintense central portion with a peripheral low signal rim. Slight ascites is present (*).
D. Sagittal gradient-echo (GE) image obtained 40 seconds after the administration of Gd-DTPA reveals strong enhancement of the peripheral portion of the mass.
E. Sagittal GE image obtained 140 seconds after demonstrates progressive centripetal enhancement (arrows).
F. At the sectioned surface of the tumor, a central whitish edematous and collagenous area (arrowheads) is visible, and this is surrounded by yellowish solid tissue, with a whitish ovarian capsule at its outermost rim.
G. Microscopic image of the cellular portion reveals an admixture of fibroblasts (arrows) and rounded vascuolated cells (curved arrows), and prominent thin-walled vessels (arrowheads) are noticeable (H&E staining, ×200).
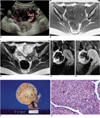
Fig. 2
Sclerosing stromal tumor of the left ovary in a 26-year-old woman.
A. Axial T1-weighted MR image demonstrates a round, left adnexal mass (arrows), with a slightly hyperintense peripheral portion relative to muscle and a homogeneous central hypointense area.
B. Coronal T2-weighted image reveals a left adnexal mass with a marked hyperintense central portion and slightly hyperintense peripheral portion. The mass is surrounded by a low-signal peripheral rim.
C. Gadolinium-enhanced MR image demonstrates strong peripheral nodular enhancement.
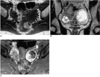
Fig. 3
Sclerosing stromal tumor of the left ovary in a 39-year-old woman.
A. Transvaginal ultrasonography reveals a homogeneously echogenic solid mass in the left adnexa. At the left of the mass, a cyst is present (*).
B. Axial T1-weighted MR image depicts a left adnexal mass which, relative to muscle, showed slightly high signal intensity. Signal void tubular structures are seen peripherally.
C. Axial T2-weighted image of the mass, demonstrating heterogeneous high signal intensity and peripheral signal-void tubular structures. Elongated cysts surround the mass (*), and a cul-de-sac contains a small amount of ascites.
D, E, F. Dynamic contrast-enhanced MR images obtained 30 seconds (D), 60 seconds (E), and 5 minutes (F) after the administration of Gd-DTPA reveal, except in small scattered areas of the cyst and cleft, marked, early, peripheral nodular enhancement, and progressive centripetal enhancement.
G. Microscopic examination reveals a pseudolobular pattern with cellular nodules separated by less cellular areas of edematous connective tissue (H&E staining, ×100).
References
1. Ihara N, Togashi K, Todo G, et al. Sclerosing stromal tumor of the ovary: MRI. J Comput Assist Tomogr. 1999. 23:555–557.
2. Matsubayashi R, Matsuo Y, Doi J, Kudo S, Matsuguchi K, Sugimori H. Sclerosing stromal tumor of the ovary: radiologic findings. Eur Radiol. 1999. 9:1335–1338.
3. Kawauchi S, Tsuji T, Kaku T, Kamura T, Nakano H, Tsuneyoshi M. Sclerosing stromal tumor of the ovary: a clinicopathologic, immunohistochemical, ultrastructural, and cytogenetic analysis with special reference to its vasculature. Am J Surg Pathol. 1998. 22:83–92.
4. Lee MS, Cho HC, Lee Y-H, Hong SR. Ovarian sclerosing stromal tumors: gray scale and color Doppler sonographic findings. J Ultrasound Med. 2001. 20:413–417.
5. Joja I, Okuno K, Tsunoda M, et al. Sclerosing stromal tumor of the ovary: US, MR, and dynamic MR findings. J Comput Assist Tomogr. 2001. 25:201–206.
6. Outwater EK, Wagner BJ, Mannion C, McLarney JK, Kim B. Sex cord-stromal and steroid cell tumors of the ovary. RadioGraphics. 1998. 18:1523–1546.
7. Chalvardjian A, Scully RE. Sclerosing stromal tumors of the ovary. Cancer. 1973. 31:664–670.
8. Jung SE, Lee JM, Rha SE, Byun JY, Jung JI, Hahn ST. CT and MR imaging of ovarian tumors with emphasis on differential diagnosis. RadioGraphics. 2002. 22:1305–1325.
9. Damjanov I, Drobnjak P, Grizelj V, Longhino N. Sclerosing stromal tumor of the ovary: a hormonal and ultrastructural analysis. Obstet Gynecol. 1975. 45:675–679.
10. Yuen BH, Robertson I, Clement PB, Mincey EK. Sclerosing stromal tumor of the ovary. Obstet Gynecol. 1982. 60:252–256.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download