Abstract
Objective
To determine whether MRI is able to demonstrate the effect of radiation synovectomy after the intra-articular injection of holmium-166-chitosan complex for the treatment of rheumatoid arthritis of the knee.
Materials and Methods
Fourteen patients aged 36-59 years were treated with 10-20 mCi of holmium-166-chitosan complex. A criterion for inclusion in this study was the absence of observable improvement after 3- or more months of treatment of the knee with disease-modifying anti-rheumatic drugs. MR images were acquired both prior to and 4-months after treatment. Clinical evaluation included the use of visual analog scales to assess pain, and the circumference of the knee and its range of motion were also determined. MR evaluation included measurement of the volume of synovial enhancement and wall thickness, the amount of joint effusion, and quantifiable scoring of bone erosion, bone edema and lymph nodes.
Rheumatoid arthritis (RA) is a chronic, progressive, inflammatory joint disorder, the primary treatment for which consists of medical regimens aimed at controlling synovial inflammation of the joint. The key to successful treatment is to begin with a program of broad therapy early in the disease, and in most patients, RA can in this way be satisfactorily controlled (1). When medical regimens have proven unsuccessful, however, surgical synovectomy has traditionally been the treatment of choice. The procedure remains, though, highly controversial (2): it is, for instance, technically difficult, if not impossible, to remove all the diseased synovium lining the joint, and alternative methods of treatment are therefore desirable.
One such method is radiation synovectomy, involving a radiopharmaceutical injection into the joint. Its intention is to destroy the inflamed synovium, in the expectation that the regenerated synovium will be disease-free and the symptoms will thereby be alleviated. The radiopharmaceuticals most commonly used have been inorganic colloids, but leakage of these from the joint has sometimes led to exposure of the liver, spleen and lymph nodes to radiation (3). Recent advances in radiopharmaceutical design and synthesis could, however, eventually lead to a new class of radiation synovectomy agents which will exhibit minimal leakage of radioactivity from the treated joint (4, 5). Leakage has been particularly difficult to quantify where the isotope used was 90Y or 32P, radioisotopes which are both pure beta emitters with no accompanying gamma emissions that might be used to quantify biodistribution and dosimetry (6). In contrast to pure beta ray emitters, 166Ho has a number of physical characteristics which make it suitable for internal radiation therapy; these include an appropriate half-life (27 hrs), high beta-energy, and low gamma-energy that permits the use of a gamma camera to monitor its distribution in the body (7).
Evaluations of the therapeutic effects of radiation synovectomy in RA cases are generally based upon improvements in measurable patient parameters, namely range of joint motion, extent of knee effusion, degree of crepitus, circumference of the knee at midpatella, and pain, and in recent years, MRI has been used to more objectively assess these effects (8-15). Measurements of synovial volume, synovial thickness, and jont effusion obtained at MRI have shown close correlation with the findings of clinical evaluation (8).
The purpose of this study was to determine whether MRI is able to demonstrate the effects of radiation synovectomy after the intra-articular injection of holmium-166-chitosan complex (166Ho-CC) for the treatment of RA of the knee.
A total of 14 female patients aged 35-59 years, treated between January and April 1999, were included in this study. All fulfilled the American College of Rheumatology (ACR) criteria (16), and the mean duration of their RA was 71.6 months. A criterion for inclusion in this study was a lack of clinical improvement after 3-or more months of treatment with disease-modifying anti-rheumatic drugs. Patients were excluded if they had undergone knee surgery or if laboratory studies revealed renal, hepatic or hematological abnormalities. During the follow-up period, medication was maintained, without dosage change, and the effect of the medicines used thus did not influence our findings. This study was approved by the Ethics Subcommittee of our hospital, and written informed consent was obtained from each patient prior to their enrollment.
166Ho is a product of the neutron activation of holmium-165, and is predominantly a beta-emitter (Emax = 1.84 MeV) with radiotherapeutic properties appropriate for therapy. It also emits gamma-ray photons (81 keV, 6.2%), detectable at scintillation imaging. Holmium solution (166Ho (NO3)3·5H20) was generated at the Korea Atomic Energy Research Institute (Taejon, Korea). In this study, we used 166Ho-CC, a combination of 166Ho and chitosan. The latter is a polymer of 2-deoxy-2-amino-D-glucose with β-1, four bonds, derived by the deacetylation of chitin (17), and was supplied by Pharmaceutical Development Lab (Dong Wha Pharm. Ind. Co. Ltd., Kyunggi-do, Korea). 166Ho-CC was injected into the knee joint via the medial or lateral routes of the suprapatellar recess after the aspiration of 1-2 mL of joint fluid. The injection volume and dose were approximately 1 mL and 10-20 mCi, respectively.
For all examinations, a 1.5-T MR imaging system (Sigma, GE Medical Systems, Milwaukee, Wis., U.S.A.) was used. Images were obtained both before and four months after the treatment of synovitis of the knee, and the following pulse sequences were used : (a) T2-weighted spin echo sagittal imaging (TR/TE=2000/70, FOV=16×16, slice thickness=4 mm with 1mm interslice gap, matrix=256×256, 1 NEX); (b) T1-weighted spin-echo axial imaging (TR/TE=620-450/14, FOV=16×16, slice thickness=4 mm with 1 mm interslice gap, matrix=256×192, 1 NEX); (c) fat-suppressed, three-dimensional spoiled gradient-echo (3D SPGR) sagittal imaging, both pre- and post-Gd-DTPA enhancement (TR/TE=21.0-23.6/2.1-3.0, FOV=16×16 to 20×20, slice thickness=1.3-1.5 mm, matrix=256×256, 1 NEX, flip angle=15).
All clinical evaluations were performed before and 4 months after radiation synovectomy. To assess knee pain, visual analog scales were used. The system, involving a form of cross-modality matching in which line length is the response continuum, is useful for the measurement of chronic pain (18), and has been reported to provide valid and reliable measurements of its intensity (19-21). The circumference of the knee at the level of the center of the patella was measured before and 4-months after radiation synovectomy for the evaluation of swelling, and the range of motion of the knee joint was also evaluated by measuring the angle of flexion and extension using a goniometer.
Extra-articular leakage of injected 166Ho-CC was evaluated in nine patients. For the analysis of gamma-scan images and radionuclide preparations, counts were corrected for the decay of 166Ho to the planned time of evaluation, based on the half-life of 166Ho. Whole-body scans were obtained using a Genesys gamma camera (ADAC, Milpitas, U.S.A.) with a high-energy collimator. The biodistribution of the injected DW-166HC was analyzed chronologically by calculating the activity counts of different regions of interest, including the knee, brain, chest and abdomen. Whole-body scans were obtained 24 hours and 8 days after injection.
The degree of synovial enhancement was determined from sagittal fat-suppressed 3D SPGR images (Fig. 1), using Scion Image (free software developed at the U.S. National Institutes of Health and modified at the Scion Corporation). Synovial enhancement volume was decided by means of the following three steps, as described previously (8, 9, 22, 23) : first, a region containing synovium was traced manually; second, a segmentation procedure was undertaken, threshold cut-off value being defined as the midpoint between the mean voxel value of the enhancing synovium minus 2 standard deviations (SD) and the mean voxel value of muscle plus 2 SDs, and mean values and SDs were obtained from multiple regions of interest; third, enhanced pixels on a segmented image were counted, and the volume was calculated. These three steps were repeated for all sagittal images relating to each patient.
Using sagittal T2-weighted images, synovial thickness was measured and summed from 18 areas of the suprapatellar pouch; anterior and posterior synovial pouch tissue was divided into midline, lateral and medial regions and then subdivided into superior, middle and inferior portions.
The amount of joint effusion was measured on T2-weighted images, where joint effusion is easily demarcated from the synovium. Using Scion Image, joint effusion was determined through three separate steps, identical to the method mentioned above. Threshold signal intensity was defined as the midpoint between the mean voxel value of the joint effusion minus 2 SDs and the mean voxel value of fat plus 2 SDs. In contrast to the evaluation of synovial enhancement, fat was selected as the comparison value due to its intermediate signal intensity on T2-weighted images. The volume of any Baker cyst present was measured using the same method.
Two radiologists (S.H.L, J.S.S) reviewed the MR images and reached their conclusions by consensus. T1-weighted axial and 3D SPGR images were used to determine the number of marginal and central erosions that involved the medial and lateral femur, medial and lateral tibia, patella, and tibio-fibular joint (Fig. 2). A lesion was defined as bone marrow edema if T2-weighted and 3D-SPGR images showed that it was poorly defined and demonstrated high signal intensity. If consecutive images indicated that a nodular lesion present in the popliteal space lacked continuity to popliteal vessels, it was regarded as a lymph node. The following scores were assigned: if one or more lesions were present at each site: 1; if the lesion was aggravated: 1.5; if the lesion had disappeared: 0; and if the lesion was newly developed: 1. The sum of the scores was obtained on every occasion.
Spearman's correlation coefficient analysis was used to assess differences between the visual analog scale readings obtained before and after radiation synovectomy, and differences in MRI findings. The paired t test was used to determine correlation between pre- and post-radiation synovectomy MR scores and clinical data. The reproducibility of the quantification of volume measurement was evaluated, intra- and inter-observer variation being expressed as mean percentage average absolute variation and percentage coefficient of variation. A Statistical Analysis System package (SPSS version 10.0) was used, and a p value of <0.05 was considered statistically significant.
Between pre- and post-radiation synovectomy, a significant decrease in the visual analog scale was noted [20.1±20.7 (range, -21 to 44)] (p < 0.05). Knee circumference decreased by 0.8 (range, -1.5 to 4.0) cm (p = 0.054), but the range of motion of the knee joint was unchanged (p > 0.1) (Table 1). In nine of the 14 patients, synovial enhancement was reduced (Fig. 1), and in five it had increased (Fig. 3), but the difference was not statistically significant (Table 2, p = 0.107). Joint effusion decreased by 8.8±12.3 ml (p < 0.05), but bony erosion, marrow edema and lymph nodes showed no change (p > 0.1). Correlation between changes in visual analog scale values and in MRI scores was not significant (p > 0.01), but there was significant correlation between the former and changes in knee circumference (p < 0.05). Among MRI scores, changes in synovial volume showed positive correlation with changes in synovial thickness (p < 0.05) and the amount of effusion (p < 0.01).
Pre- and post-radiation synovectomy images indicated that the lateral femur was the most frequent site of bony erosion (score of 27), followed by the medial tibia (21.5), medial femur (20.5), lateral tibia (7), tibio-fibular joint (6) and patella (1). Bone marrow edema was observed most frequently in the medial femur (15), medial tibia (9), lateral femur (7.5), lateral tibia (5.5) and patella (2).
Twenty-four hours after the injection of 166Ho-CC, almost all radioactivity was still retained in the knee joint ; less than 1% had spread to other tissues. Eight days after injection, activity distribution rates had increased in the brain, lungs, abdomen and pelvic area to 1.5%, 2.0%, 1.9%, and 1.9%, respectively.
In this study, high intra- and inter-observer reproducibility were demonstrated. For observer A, mean percentage average absolute variation was 0.5 (range, 0.16-2.1), and the percentage coefficient of variation was 1.3% (0.1-1.8). Inter-observer mean percentage coefficient of variation was 0.5% (0.1-0.9).
Radiation synovectomy is a locally acting treatment for chronic refractory synovitis and is an alternative to intra-articular injection of corticosteroids and other systemic pharmacological treatments. Radionuclide treatment has advantages over external radiotherapy: because most radionuclides currently used are beta emitters, which have properties of high linear energy transfer and rapid fall-off, there are no adverse effects on adjacent bones and soft tissues.
An ideal radiation synovectomy agent should have the following four characteristics (6, 25): first, the radionuclide should have beta-particle energy sufficient to penetrate and ablate the enlarged area of synovial tissue but not so great as to damage underlying articular cartilage or overlying skin. Any accompanying radiation should not generate an unacceptable, extraneous dose. Second, the radionuclide should be attached to a particle that is sufficiently small to be phagocytized but not so small as to leak from the joint before being phagocytized; the appropriate size range is usually thought to be 2-5 µm. The binding between the radionuclide and particle should be irreversible throughout the course of the radiotherapy; the timespan is determined by the physical half-life of the particular isotope employed. Third, the particle should be biodegradable; persistence in the joint of non-biodegradable materials can itself give rise to granulomatous tissues. Fourth, and finally, any biologically induced degradation of the agent should ideally release the radionuclide in a chemical form that rapidly egresses from the body.
The accepted biological mechanism by which these agents function involves their rapid phagocytosis by synoviocytes, their even distribution over the surface of the synovium, and the irradiation of pathologic synovial tissue.
The primary disadvantage of this procedure is the unacceptable radiation doses delivered to non-target organ systems due to leakage of radioactive material from the cavity. Leakage can, however, potentially be avoided in two ways: by using large carrier particles or aggregates, and by choosing a radioisotope that has a short half-life, thus allowing almost all radioactivity to decay before leakage occurs (25).
166Ho-CC was initially introduced as an intratumoral administration agent (7), and if injected locally into the liver or a solid tumor, it precipitates, thus preventing systemic distribution of 166Ho. It is because chitosan dissolves in dilute mineral and organic acids, but precipitates at a pH of above 6.0, that distribution does not occur (17). After we administered 166Ho-CC, radioactive concentrations in the blood were low and cumulative urinary and fecal excretions over a period of 0-72 hrs were 0.53% and 0.54%, respectively. Suzuki et al. (7) has proved the suitability of 166Ho-CC for local injection by showing that 24 hours after injection, less than 1% of the total injected amount was detectable in tissues other than those at the injection site. 166Ho-CC leaks only minimally because in tissues it is transformed into a gel, and has an appropriate half-life. In toxicity evaluations after the intravenous injection of 166Ho-CC, the major effects of injections of 1 mCi/kg were limited to the spleen, and the hematological parameters were reversible by day 14 (28). Our report is the first to describe the MRI evaluation of DW-166HC radiation synovectomy in patients with RA. Information regarding biodistribution, doses, and adverse effects after intra-articular injection has been detailed by Song et al. (27).
Several radionuclides are used for radiation synovectomy, namely 90Y, 32P, 186Re, 169Er, 165Dy, and 166Ho. Because of the deep penetration capability of its energetic beta particles (mean tissue penetration of 3.6 mm), 90Y has been most commonly used to treat the rheumatoid knee, while rhenium-186 and erbium-169 agents are also recommended for intermediate-sized joints (mean tissue penetration of 1.2 mm) and the smallest joints (mean tissue penetration of 0.3 mm), respectively (6). We believe that 166Ho has several advantages over other radionuclides. First of all, it can be produced in a simple way from 165Ho, a naturally abundant element, and is less expensive than 90Y; second, because it can emit the gamma rays necessary for image acquisition, it is also suitable for quantitative dosimetric studies; third, it has a half-life of 26.8 hours; lastly, it has an adequate penetration range for synovial ablation, while avoiding damage to adjacent cartilage or bone. 166Ho deposits ninety percent of its energy within an area 2.1 mm in diameter, while the remainder is deposited within an area measuring 2.1-8.7 mm (28).
The absorbed dose relative to depth required for successful radiation synovectomy has not been established. In fact, the rationale for choosing a particular dose of a given radionuclide in a given joint remains obscure, and most published studies have relied on historically derived, empirical dosimetry (28). In patients with RA, 90Y is the most commonly used agent for radiation synovectomy of the knee, and the most commonly used dosage is 5 mCi. Deutsch et al. (6) stated that at this dosage, 91% of patients showed a good response. 90Y delivers an absorbed dose (28 mGy/MBq) higher than that delivered by 166Ho (8.7 mGy/MBq) (29), and the therapeutic dosage of 166Ho must therefore be at least three times greater than that of 90Y.
In RA, the synovium becomes inflamed, and the synovial mass consists of vascular congestion, edema and cellular infiltration as well as hyperplasia of the synovial lining and pannus tissue formation (11, 30). The histologic changes occurring after radiation synovectomy include the regression of destructive pannus, and the reduction of cellular infiltration and synovial sclerosis (31). The severity of the disease might possibly be determined by measuring the volume of synovial inflammation.
Several investigators have described the use of MRI for quantification of the synovial volume of the knee joint (10, 11-13, 32). Although there is no consensus as to the optimal MRI method for synovial volume measurement, we used fat-suppressed, enhanced 3D SPGR imaging, which has been previously described and permits a thin slice setting and high tissue contrast between enhancing synovium and non-enhancing tissue (8, 9, 33). Synovial volume measurement has proven useful for evaluating the therapeutic effectiveness of antirheumatic drugs (10, 12). Ostergaard et al. (10) reported that the duration of clinical remission in patients with RA showed significant inverse correlation to pre-treatment synovial volume. They found that at relapse, synovial volume had increased roughly to pretreatment levels, but effusion volumes remained lower than before treatment, and thus insisted that synovial volume measurement could play a more important role in the assessment of treatment outcome.
Only one published report has described the usefulness of synovial volume measurement after radiation synovectomy (90Y and triamcinolone) (11), noting that synovial volume tended to decrease, but not to a statistically significant extent. Our results showed a similar tendency after treatment with 166Ho-CC, and the decrease was also statistically insignificant.
As a means of evaluating the outcome of radiation synovectomy, investigators have used MR imaging to measure synovial thickness (14, 34). Thickness was found to be reduced by 4.4-7.7 mm at one-year follow-up after 165Dy treatment (34), and by 1.0-2.3 mm at six-month followup after 90Y treatment (14). However, our results demonstrated no significant change in synovial thickness, which had decreased from 3.8 mm to 3.7 mm at follow-up four months after treatment. We believe, however, that because changes in synovial effusion may lessen the accuracy of synovial thickness measurement, the measurement of synovial volume is a more reliable indicator. It is conceivable that as synovial effusion decreases, proliferated synovial tissue can become folded from an unfolded or effaced state.
Joint effusion has been thought to be a therapeutic response indicator (14); as measured by joint aspiration (14) or MRI (10, 11), it has shown reductions after radiation synovectomy ; good correlation was found between the aspirated volume of synovial fluid and the volume of fluid calculated after MRI (11). In our study, the volume of effusion measured by MRI was significantly lower after therapy, and, as was expected, correlated well with the circumference of the knee, an indirect indicator of joint swelling. On the other hand, the amount of fluid in Baker cysts showed no significant change. We believe that therapeutic effect may depend on communication between a Baker cyst and the joint, though our study did not investigate whether Baker cysts communicated with the knee joint.
Two common alterations occurring in the juxta-articular bones in RA are osteoporosis and marginal erosion. The latter usually develops near synovial pockets and on bare areas that do not possess protective cartilaginous coats (35), and may represent a very early manifestation of the disease, occurring one or two years prior to the onset of joint symptoms. In this study, the bony erosion score tended to increase even after radiation synovectomy, and for this there are three possible reasons. One is that as explained by de Carvalho et al. (36), areas of bony erosion may remain static for long periods of time or even become bigger as more widespread osseous damage becomes manifest. The second possible explanation is the fact that unless erosive lesions communicate with the joint, intruded synovial tissue within the lesions cannot be reached by injected radiopharmaceuticals (37, 38). Finally, although the amount of synovial tissue in an eroded area is reduced over a given period, bone erosion may not be refilled with bone or marrow tissue.
The occurrence of bone marrow edema has not been fully described, and the significance of its presence in RA has been poorly investigated. In our study involving RA patients, bone marrow edema was a common finding, though other investigators have found it to be a rare manifestation (39). This discrepancy may result from differences in the duration of disease: in that earlier study, the mean duration of knee synovitis was about ten weeks, whereas all synovitis cases in our study were chronic. Bone marrow edema was found most frequently in the medial femur, but bone erosion had occurred in the lateral femur, a finding which corroborates the fact that bone marrow edema is associated not only with bone erosion but also with conditions such as periarticular osteoporosis, hyperemia, co-existing osteoarthritis, and reflex sympathetic dystrophy superimposed on the rheumatoid process (35). It has been postulated in only one study that marrow edema reduction is associated with remission after disease modifying anti-rheumatic drug therapy (40); our results, however, showed that bone marrow edema did not change significantly after radiation synovectomy. We speculated that marrow edema reduction would ensue if synovial inflammation was reduced, but this did not occur during our follow-up period, and for the evaluation of marrow edema in RA, further study is thus required.
Lymph nodes, which in this study occurred frequently in the popliteal fossa, were rarely observed at MR imaging for the evaluation of internal derangement of the knee. If seen at MRI, they would have been abnormal. In this study, lymph nodes showed no interval change after treatment. Kojima et al. have reported the presence of reactive proliferative lymph nodes in RA (41), describing histological findings of follicular hyperplasia with active germinal centers, and polyclonal plasma cell infiltration of the interfollicular area. The relationship between lymph node size and disease activity is one that future studies should address.
Certain investigators have reported the usefulness of MR perfusion studies in the evaluation of RA, analysing synovial enhancement in terms of its degree and the maximal gradient of increased signal intensity (11), the maximal enhancement ratio (14), the intensity of enhancement (34), the time to maximum synovial lining signal ratio, and the maximum synovial lining signal ratio (13). To determine the usefulness of the quantification of synovial enhancement, further investigation is required.
Our study suffers several limitations. One is that it is cross-sectional, demonstrating clinical and MR imaging changes occurring during a 4-month follow-up period after 166Ho-CC radiation synovectomy. There are no comparison or control data. Other limitations are the small number of patients and the short duration of follow-up. In the future, long-term follow-up data should be collected from the same patients, and a larger patient population should be studied. From a technical point of view, MR contrast agent may have leaked into a joint within the period of image acquisition, affecting MRI measurements and perhaps leading to the overestimation of synovial volume.
In conclusion, the decreased joint effusion observed at 4-month follow-up resulted from radiation synovectomy of the rheumatoid knee by means of intra-articular injection of 166Ho-CC. Changes in the volume of synovial enhancement should be further followed up.
Figures and Tables
Fig. 1
MR images of a 50-year-old woman with rheumatoid arthritis of the knee.
A. Postcontrast fat-suppressed, three-dimensional spoiled gradient-echo sagittal image (TR/TE=21.1/2.2, flip angle=15°) shows a highly enhanced pannus (arrowheads). Joint effusion (arrow) is seen, with low signal intensity at the suprapatellar recess before treatment involving the intra-articular injection of 166Ho-chitosan complex.
B. MR image obtained four months after treatment shows that the size of the pannus and joint effusion have decreased.
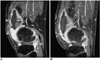
Fig. 2
MR images of a 37-year-old woman with rheumatoid arthritis of the knee.
A. Postcontrast, fat-suppressed, three-dimensional spoiled gradient-echo sagittal image (TR/TE = 21.1/2.2, flip angle=15°) obtained before the intra-articular injection of 166Ho-chitosan complex depicts extensive bone marrow edema in the lateral condyle of the femur and tibia (arrows). Enhanced synovial tissue is seen in the infrapatellar region and posterior joint space (arrowheads), and in the suprapatellar pouch, joint effusion is apparent (double arrow).
B. MR image obtained four months after treatment shows that bone marrow edema has almost disappeared. Bony erosion still remains, however, and is clearly demarcated in the subchondral bone of the lateral tibia (arrow). Joint effusion and the size of the pannus have both decreased.
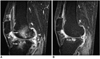
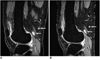
Fig. 3
MR images of a 54-year-old woman with rheumatoid arthritis of the knee.
A. Postcontrast, fat-suppressed, three-dimensional spoiled gradient-echo sagittal image (TR/TE = 21.1/2.2, flip angle=15°) obtained before the intra-articular injection of 166Ho-chitosan complex depicts lymph nodes in the popliteal region (arrows).
B. MR image obtained 4-months after treatment shows that the size and number of the lymph nodes are unchanged (arrows).
Acknowledgements
The authors wish to thank Dongjun Kim for help with manuscript preparation and editorial assisstance. The Korea Atomic Energy Research Institute and Dong Wha Pharm. Ind. Co., Ltd. provided DW-166HC.
References
1. Zuckerman JD, Sledge CB, Shortkroff S, Venkatesan P. Treatment of rheumatoid arthritis using radiopharmaceuticals. Int J Rad Appl Instrum B. 1987. 14:211–218.
2. Sledge CB, Zuckerman JD, Shortkroff S, et al. Synovectomy of the rheumatoid knee using intra-articular injection of dysprosium-165 ferric hydroxide macroaggregates. J Bone Joint Surg Am. 1987. 69:970–975.
3. Sledge CB, Noble J, Hnatowich DJ, Kramer R, Shortkroff S. Experimental radiation synovectomy by 165-Dy ferric hydroxide macroaggregate. Arthritis Rheum. 1977. 20:1334–1342.
4. Makela O, Penttila P, Kolehmainen E, Sukura A, Sankari S, Tulamo RM. Experimental radiation synovectomy in rabbit knee with holmium-166 ferric hydroxide macroaggregate. Nucl Med Biol. 2002. 29:593–598.
5. Brodack JW, Chinen LK, Deutsch E, Deutsch KF. Studies on the radiolabeling of hydroxyapatite particles for use as radiation synovectomy agents. J Nucl Med. 1992. 33:980.
6. Deutsch E, Brodack JW, Deutsch KF. Radiation synovectomy revisited. Eur J Nucl Med. 1993. 20:1113–1127.
7. Suzuki YS, Momose Y, Higashi N, et al. Biodistribution and kinetics of Ho-166-chitosan complex in rats and mice. J Nucl Med. 1998. 39:2161–2166.
8. Palmer WE, Rosenthal DI, Schoenberg OI, et al. Quantification of inflammation in the wrist with gadolinium-enhanced MR imaging and PET with 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology. 1995. 196:647–655.
9. Huh YM, Suh JS, Jeong EK, et al. Role of inflamed synovial volume of the wrist in defining remission of rheumatoid arthritis with gadolinium-enhanced 3D-SPGR imaging. J Magn Reson Imaging. 1999. 10:202–208.
10. Ostergaard M, Stoltenberg M, Gideon P, Sorensen K, Henriksen O, Lorenzen I. Changes in synovial membrane and joint effusion volumes after intra-articular methylprednisolone: quantitative assessment of inflammatory and destructive changes in arthritis by MRI. J Rheumatol. 1996. 23:1151–1161.
11. Creamer P, Keen M, Zananiri F, et al. Quantitative magnetic resonance imaging of the knee: a method of measuring response to intra-articular treatments. Ann Rheum Dis. 1997. 56:378–381.
12. Ostergaard M, Hansen M, Stoltenberg M, et al. Magnetic resonance imaging-determined synovial membrane volume as a marker of disease activity and a predictor of progressive joint destruction in the wrists of patients with rheumatoid arthritis. Arthritis Rheum. 1999. 42:918–929.
13. Clunie G, Hall-Craggs MA, Paley MN, et al. Measurement of synovial lining volume by magnetic resonance imaging of the knee in chronic synovitis. Ann Rheum Dis. 1997. 56:526–534.
14. Alonso-Ruiz A, Perez-Ruiz F, Calabozo M, et al. Efficacy of radiosynovectomy of the knee in rheumatoid arthritis: evaluation with magnetic resonance imaging. Clin Rheumatol. 1998. 17:277–281.
15. Konig H, Sieper J, Wolf KJ. Rheumatoid arthritis: evaluation of hypervascular and fibrous pannus with dynamic MR imaging enhanced with Gd-DTPA. Radiology. 1990. 176:473–477.
16. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988. 31:315–324.
17. Muzzarelli R, Baldassarre V, Conti F, et al. Biological activity of chitosan: ultrastructural study. Biomaterials. 1988. 9:247–252.
18. Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983. 17:45–56.
19. Ohnhaus E, Adlev R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975. 1:379–384.
20. Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976. 2:175–184.
21. Revill SI, Robinson JO, Rosen M, Hogg MI. The reliability of a linear analogue for evaluating pain. Anaesthesia. 1976. 31:1191–1198.
22. Disler DG, Marr DS, Rosenthal DI. Three-dimensional reconstruction: accuracy of volume measurements of phantoms, with clinical trial. Invest Radiol. 1994. 29:739–745.
23. Michael AP, David GD, Thomas RM, et al. Articular cartilage volume in the knee: semiautomated determination from three-dimensional reformations of MR images. Radiology. 1996. 198:855–859.
24. Lee JD, Park KK, Lee MG, et al. Radionuclide therapy of skin cancers and Bowen's disease using a specially designed skin patch. J Nucl Med. 1997. 38:697–702.
25. Noble J, Jones AG, Davies MA, Sledge CB, Kramer RI, Livni E. Leakage of radioactive particle systems from a synovial joint studied with a gamma camera: its application to radiation synovectomy. J Bone Joint Surg Am. 1983. 65:381–389.
26. Lee WY, Moon EY, Lee J, et al. Toxicities of 166Ho-chitosan in mice. Arzneimittelforschung. 1998. 48:300–304.
27. Song JS, Suh CH, Park YB, et al. A phase I/IIa study on intra-articular injection of Holmium-166-chitosan complex for the treatment of knee synovitis of rheumatoid arthritis. Eur J Nucl Med. 2001. 28:489–497.
28. Johnson LS, Yanch JC, Shortkroff S, Barnes CL, Sitzer AI, Sledge CB. Beta-particle dosimetry in radiation synovectomy. Eur J Nucl Med. 1995. 22:977–988.
29. Mumper RJ, Ryo UY, Jay M. Neutron-activated holmium-166-poly (L-lactic acid) microspheres: a potential agent for the internal radiation therapy of hepatic tumors. J Nucl Med. 1991. 32:2139–2143.
30. Schumacher HR Jr. Kelley WN, Ruddy S, Harris ED, Sledge CB, editors. Synovial fluid analysis and synovial biopsy. Textbook of rheumatology. 1997. 5th Ed. Philadelphia: Saunders;609–625.
31. Harbert JC. Harbert JC, Eckelman WC, Neumann RD, editors. Radionuclide therapy in joint disease. Nuclear medicine: diagnosis and therapy. 1995. New York: Thieme;1093–1109.
32. Waterton JC, Ajanayagam V, Ross BD, Brown D, Whittemore A, Johnstone D. Magnetic resonance methods for measurement of disease progression in rheumatoid arthritis. Magn Reson Imaging. 1993. 11:1033–1038.
33. Polisson RP, Schoenberg OI, Fischman A, et al. Use of magnetic resonance imaging and positron emission tomography in the assessment of synovial volume and glucose metabolism in patients with rheumatoid arthritis. Arthritis Rheum. 1995. 38:819–825.
34. Pirich C, Schwameis E, Bernecker P, et al. Influence of radiation synovectomy on articular cartilage, synovial thickness and enhancement as evidenced by MRI in patients with chronic synovitis. J Nucl Med. 1999. 40:1277–1284.
35. Resnick D, Niwayama G. Resnick D, editor. Rheumatoid arthritis and the seronegative spondyloarthropathies: radiographic and pathologic concepts. Diagnosis of bone and joint disorders. 1995. 3rd ed. Philadelphia: Saunders;807–860.
36. de Carvalho A, Graudal H, Jorgensen B. Radiologic evaluation of the progression of rheumatoid arthritis. Acta Radiol Diagn (Stockh). 1980. 21:115–121.
37. Gubler FM, Maas M, Dijkstra PF, de Jongh HR. Cystic rheumatoid arthritis: description of a nonerosive form. Radiology. 1990. 177:829–834.
38. Moore EA, Jacoby RK, Ellis RE, Fry ME, Pittard S, Vennart W. Demonstration of a geode by magnetic resonance imaging: new light on the cause of juxta-articular bone cysts in rheumatoid arthritis. Ann Rheum Dis. 1990. 49:785–787.
39. McGonagle D, Gibbon W, O'Connor P, Green M, Pease C, Emery P. Characteristic magnetic resonance imaging entheseal changes of knee synovitis in spondyloarthropathy. Arthritis Rheum. 1998. 41:694–700.
40. Lee J, Lee SK, Suh JS, Yoon M, Song JH, Lee CH. Magnetic resonance imaging of the wrist in defining remission of rheumatoid arthritis. J Rheumatol. 1997. 24:1303–1308.
41. Kojima M, Hosomura Y, Itoh H, et al. Reactive proliferative lesions in lymph nodes from rheumatoid arthritis patients. a clinicopathological and immunohistological study. Acta Pathol Jpn. 1990. 40:249–254.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download