Abstract
Objective
To compare the in-vitro efficiency of a hypertonic saline (HS)-enhanced bipolar radiofrequency (RF) system with monopolar RF applications by assessing the temperature profile and dimensions of RF-created coagulation necrosis in bovine liver.
Materials and Methods
A total of 27 ablations were performed in explanted bovine livers. After placement of two 16-gauge open-perfused electrodes at an interelectrode distance of 3 cm, 5% HS was instilled into tissue at a rate of 1 mL/min through the electrode. Seventeen thermal ablation zones were created in the monopolar mode (groups A, B), and ten more were created using the two open-perfused electrodes in the bipolar mode (group C). RF was applied to each electrode for 5 mins (for a total of 10 mins, group A) or 10 mins (for a total of 20 mins, group B) at 50W in the sequential monopolar mode, or to both electrodes for 10 min in the bipolar mode (group C). During RF instillation, we measured tissue temperature at the midpoint between the two electrodes. The dimensions of the thermal ablation zones and changes in impedance and wattage during RFA were compared between the groups.
Results
With open-perfusion electrodes, the mean accumulated energy output value was lower in the bipolar mode (group C: 26675 ± 3047 Watt·s) than in the monopolar mode (group A: 28778 ± 1300 Watt·s) but the difference was not statistically significant (p > 0.05). In the bipolar mode, there were impedance rises of more than 700 Ω during RF energy application, but in the monopolar modes, impedance did not changed markedly. In the bipolar mode, however, the temperature at the mid-point between the two probes was higher (85℃) than in the monopolar modes (65℃, 80℃ for group A, B, respectively) (p < 0.05). In addition, in HS-enhanced bipolar RFA (group C), the shortest diameter at the midpoint between the two electrodes was greater than in either of the monopolar modes: 5.4 ± 5.6 mm (group A); 28.8 ± 8.2 mm (group B); 31.2 ± 7.6 mm (group C) (p < 0.05)
During the past few years, percutaneous radiofrequency ablation (RFA) has become accepted as an alternative interventional therapy, especially for the treatment of primary and secondary liver tumors (1-4). However, although the dimensions of zones of ablation for different treatment parameters and different systems have been described in the literature, a thermally induced ablation zone generated by a commercial monopolar ablation system is generally not greater than 4 cm in diameter (1, 5, 6). This limitation of the monopolar radiofrequency (RF) technique often results in failure to create a safety margin of clinically relevant size (< 3-5 cm in diameter) around a treated tumor (5-7).
Several methods by which lesion size might be augmented, and efficacy thus increased, have been investigated. These include the pulsed technique (8, 9), saline-enhanced RFA (10-12), bipolar RFA (13, 14), and perfusion-mediated RFA (15, 16). Bipolar RFA, in which two electrodes are used, has been shown to create larger lesions than monopolar ablation (13, 14). Gangi et al. (unpublished data, ECR 2003) recently demonstrated that hypertonic saline (5%)-enhanced RFA using a commercially available system (Elektrotom HiTT® 106; Berchtold Medizinelektronik, Tuttlingen, Germany) could more quickly create round and regular lesions. To prove the clinical efficacy of bipolar RFA, however, it is necessary to compare the efficiency of HS-enhanced bipolar RFA with overlapping sequential monopolar RFA in creating thermally-mediated coagulation necrosis. In this context, we present the results of our systematic evaluation of these two modes, in which the dimensions of ablation zones in liver tissue and the temperature at the midpoint between the two electrodes were measured.
RF ablation (RFA) was performed in ten freshly excised bovine livers weighing, on average, 1,200 g. The liver was cut into several 10×10-cm blocks which were dipped into a 50×20-cm saline-filled bath. Using two 16-gauge open-perfused electrodes (Berchtold Medizinelektronik), 17 thermal ablation zones were created in the sequential monopolar mode (groups A [5+5 mins] and B [10+10 mins]), and a further ten ablation zones were created using the two open-perfused electrodes in the bipolar mode (group C, 10 mins). The tips of the electrodes were advanced at least 3 cm into the target tissue, and to continuously measure local tissue temperature during the procedure, a thermocouple was inserted midpoint between them. Tissue impedance was monitored by circuitry incorporated into the generator.
The RFA system used for monopolar RFA comprised a 375-kHz generator (Elektrotom HiTT® 106, Berchtold Medizinelektronik), used at 50 watts, and two 16-gauge open-perfused electrodes, each in the form of a needle with tip exposure of 2 cm. A saline solution of 5% NaCl was used as perfusion liquid, and with flow adjusted to 1 mL/min, RF energy was applied for 5 or 10 mins. If impedance change was moderate, within a range of 100 to 350Ω, RF power was stabilized by the control mechanism. At above 700Ω, a power decrease to 5 watts was enforced. For bipolar RFA, two electrodes were used, one active and one dispersive.
For monopolar RFA, the distance between the electrodes and the dispersive metallic pad was set at 35 cm. Following the procedure of a previous experimental study by Gangi et al. (unpublished data, ECR 2003), two electrodes were placed in the liver, 3 cm apart, through an acryl plate containing multiple holes at 5-mm intervals. RF power was increased manually to 50 watts, and RF energy was applied sequentially for five or ten minutes in the monopolar mode by changing the current flow to the second probe just after ablation with the first. In this mode, current flows from one electrode to a dispersive metallic pad (Figs. 1A, B).
For bipolar RFA, two open-perfused electrodes were placed in liver tissue, 3 cm apart and without a dispersive pad, and attached to the Berchtold® generator (Fig. 1C). In this mode, current flows from one electrode to the other. Again following Gangi's procedure, two electrodes were placed in the liver, 3 cm apart, through an acryl plate with multiple holes at 5 mm intervals. Using an infusion pump (Pilotec IS; Frensenius Vial SA, Breanzins, France) the 5% hypertonic saline was infused at a rate of 1 mL/m through an open perfused electrode.
The technical aspects of RFA including impedance and wattage changes, tissue temperature at midpoint between the two electrodes, and the dimensions of the RF-coagulated area were compared for each technique.
The liver blocks containing lesions were dissected along the axis along which the electrode was inserted, and the central white area of the RF-induced ablation zone was found to correspond to the necrotic zone (17). For macroscopic examination, two observers used calipers to measure, in the central white area of coagulation necrosis in each pathologic specimen, the overlapping width, the longest diameter of each ablation sphere along the electrode, and the shortest diameter at the midpoint between the two electrodes (Fig. 2). For each set of measurements, a consensus was reached.
To compare the dimensions of the thermal ablation area and technical parameters in the three groups, one-way analysis of variance by means of the Scheffe test (post-hoc testing) was performed using SPSS 9.0 computer software (SPSS Inc., Chicago, Ill., U.S.A.) In addition, using the Instat program (GraphPad Software, Inc., San Diego, Cal., U.S.A.) (18), the Kruskal-Wallis test (post-hoc testing) was performed to compare the temperature at the midpoint between the two electrodes. For all statistical analyses, a p value of less than 0.05 was considered statistically significant.
Impedance: In groups A and B (monopolar mode), impedance values during RF application showed moderate variation with the range of 100Ω to 350Ω. In group C (bipolar mode), however, mean initial impedance was less than 100Ω, but rose intermittently to more than 700Ω within approximately 4-6 mins of the instillation of RF energy.
Energy output: The mean accumulated energy output was 28778±1300 Watt·s in group A, and 26675±3047 Watt·s in group C, a statistically insignificant difference (p > 0.05). In group B, the mean corresponding output value was 52904±4739 Watt·s.
The graphs showing the mean temperature at the midpoint between the two electrodes appear in Fig. 3. In groups A and B, mean final-temperature values were 65±10℃ and 81±8℃, respectively, and in group C, 85±13℃. This value was higher in groups B and C than in group A (p < 0.05).
After RFA, a well-defined area with central white discoloration was seen in the liver section of the ablated zones. The maximum overlapping width, measured in gross specimens of the three groups, was as follows: 40.4±7.8 mm in group A, 49.8±4.1 mm in group B, and 49.2±6.6 mm in group C (p < 0.05) (Table 1). In addition, the mean shortest coagulation diameters at the midpoint between the two electrodes were largest in group C: 5.4±5.6 mm in group A, 28.8±8.2 mm in group B, and 31.2±7.6 mm in group C. In groups B and C, the mean overlapping widths of the ablated spheres, measured along the two electrodes, and the shortest diameter, were larger than in group A (p < 0.05) (Fig. 4). Compared with monopolar RFA (group A), bipolar RFA (group C) therefore tended to produce oval-shaped coagulation with less prominent waist formation at the midpoint between the two electrodes (Table 1). Furthermore, bipolar RFA took less time than was required by monopolar RFA, group B, to produce similar coagulation necrosis, even though the energy output of the former was less than that of the latter (28778±1300 Watt·s vs. 26675±3047 Watt·s). Per unit of electrical energy used, bipolar RFA was thus more efficient.
RFA using monopolar techniques has been used for the treatment of focal liver malignancies, renal tumors, and breast tumors (1, 2). RFA has great potential, but the use of monopolar RF techniques and currently available RF devices fails to create a thermally-mediated zone of ablation which is 3-5 cm in diameter and thus large enough to cover the target tumor. This limitation is related to tissue desiccation and charring at the electrode tip, phenomena attributed primarily to the rapid increase in electrical impedance and marked alterations in of electrical current that typically occur when tissues are heated to greater than 100℃ (19). To overcome this problem, one of the most effective approaches, since it increases electrical conductance and thermal conductivity, is infusion of a saline solution (11, 12, 19). In addition, several studies have demonstrated that bipolar RFA can create larger lesions than monopolar RFA: in the bipolar mode, the current is more concentrated between the probes than in monopolar mode, in which current travels outward toward a dispersive pad (13, 14). In this study, therefore, we hypothesized that a bipolar technique combined with HS infusion could more efficiently create thermal lesions than could monopolar modes, and compared the in-vitro efficiency of the former and the latter.
Our results showed that the bipolar saline-enhanced method created larger lesions than the monopolar method. Interestingly, however, the energy accumulated during RFA was less in the bipolar mode than in monopolar modes. The larger lesions created using less accumulated RF energy output in the bipolar mode than in monopolar modes could, therefore, be attributed to the greater energy efficiency of the former, which might be explained by the high concentration of current between the two electrodes and the thermodynamic effects observed in the bipolar mode (20, 21). In monopolar modes, heat is diverted from the ablation site in all directions. In the bipolar mode, in contrast, one electrode is thermally shielded by the opposing second electrode, which also actively heats the tissue in its proximity (21); there is, furthermore, since heat is trapped between the two electrodes and higher temperatures are thus achieved, less cooling in the direction of the collateral electrode than is the case with monopolar ablation. With the probes in the same positions, this results in a lesion larger than the two lesions produced sequentially by monopolar ablation.
In our study, impedance suddenly rose rapidly during bipolar RFA to more than 700Ω, resulting in reduced power output (26675±3047 Watt·s) compared to the monopolar mode (28778±1300 Watt·s), a fact which can be explained by the balance between the current in the proximity of the electrode, and tissue temperature. While current does contribute to the increased size of a thermal ablation zone up to 100℃ (without causing tissue boiling), a current which is too high might induce such boiling due to rapidly increased tissue impedance. This phenomenon could be supported by the rapid rise in tissue temperature occurring earlier (approximately four minutes after the instillation of RF energy), compared with the monopolar mode (Fig. 3). In order to minimize impedance rises during bipolar RFA, current density between the electrodes should therefore be decreased to within the range within which current causes increases in tissue temperatures, without boiling. We speculate that changing the electrical conductance of the tissue by infusing NaCl solution could be a good solution, and there is a much room for further optimization of the concentration of hypertonic saline and the infusion rate.
During monopolar RFA using an open-perfused electrode, however, the impedance did not change rapidly, suggesting that HS-enhanced monopolar RFA using a perfusion electrode could effectively prevent desiccation or charring around the electrodes. The increased electrical conductance induced by the infusion of hypertonic saline through an open perfusion electrode could prevent desiccation, but to enlarge the zone of ablation using a monopolar Berchtold® system, it appears necessary to increase total current deposit around the electrode by increasing the duration of RF application or maximum power output, as with other commercially available RF systems. In this present study, the greater efficiency of the bipolar RF mode using the Berchtold® system was demonstrated, but to determine whether this is so where other monopolar RF systems with larger maximum power output (including Radionics™, Radiotherapeutics™ and RITA® systems) are used at their maximum outputs, further comparative studies are needed.
Since McGahan et al. (13) reported that bipolar RF ablation appeared to offer increased tissue coagulation in fresh bovine liver, compared with the monopolar technique, reports have described the greater efficiency of bipolar RF ablation in this aspect (14, 20). In our study, bipolar RF application for 10 minutes created ablation zones with a mean width of 49.2±6.0 mm and a minimum diameter of 31.2±7.6 mm, dimensions which are comparable with those of a previous in-vitro experimental study involving bovine liver, in which lesion width was approximately 1-1.5 cm greater than needle length, and width was approximately 1.5-2 cm greater than inter-needle distance (13). In an in-vivo study by Curley et al. (14), however, the diameter of bipolar RF-induced ablation zones ranged from 2.0 to 2.5 cm, which was much less than in our study. We speculate that this difference could be attributed to perfusion-mediated cooling in in-vivo liver tissue.
Jang et al. (22) recently reported that simultaneous RFA using two-needle electrodes could produce greater coagulation necrosis than two successive applications of monopolar ablation using single-needle electrodes. However, the theoretical disadvantage of the simultaneous monopolar mode is that there is a large area of relatively constant electric potential between the two electrodes, resulting in reduced power deposition in this area due to the small field gradient between the electrodes, and less desirable deep waist formation midway between the electrodes (20). Bipolar RFA, on the other hand, has the potential to create more concentrated current flow between the two electrodes, and more extensive well-defined RF-induced coagulation necrosis is therefore a possibility. Since focal liver lesions are usually round or oval shaped, this ability of bipolar RFA to produce a well-defined oval-shaped lesion could be valuable for clinical RF application in cases involving liver tumors, and could also be beneficial when the target tumor lies close to structures which are vulnerable to heat, such as the bile duct, or bowel.
Although hypertonic saline-enhanced bipolar RFA more efficiently created coagulation necrosis than did monopolar RFA, it has certain drawbacks. One of these is that only two electrodes can be used at the same time, and another is that all the current originating from one electrode must also enter the second electrode (20). When using the bipolar mode there is no way to independently control the amount of heat generated in the vicinity of each electrode. When the degree of cooling at the site of each electrode is different due to differences in perfusion, one electrode thus reaches a higher temperature than the other and this can lead to boiling and a rapid rise in impedance.
Our experimental study suffers certain limitations. First, it was performed in-vitro, and the extent to which the results can be transposed to human liver is limited. In living tissue, there is a cooling "sink" containing, for example, blood vessels, and within the treated area, rapid heat exchange can thus arise (13). Furthermore, all ablations involved normal liver parenchyma, not tumor tissue. Despite the foregoing, however, our model provides a reliable basis for a comparative study of the efficiency of different RF modes. Second, following the procedure employed in an earlier study, we tested only 5% NaCl solution infused at a rate of 1 mL/min. Given that sudden rapid impedance rises occur during bipolar RFA, we believe that further experimental studies to determine the optimun concentration and amount of hypertonic saline solution are warranted. Third, and last, tissue temperature was measured at only one site.
In conclusion, using an open-perfused. electrode to create thermal coagulation, bipolar RFA performed better than monopolar ablation and during a single application may kill more tumor cells more quickly.
Figures and Tables
Fig. 1
General setting in monopolar and bipolar radiofrequency ablation in an in-vitro bovine liver model.
A. Berchtold® radio-frequency system in monopolar mode (groups A and B). An open perfused electrode and injector were used for the continuous injection of hypertonic saline. Impedance, accumulated energy and power were continuously monitored by a generator. A thermocouple was inserted 15 mm from an electrode.
B. Berchtold® radio-frequency system in bipolar mode (group C). Two pumps were employed to infuse hypertonic saline solution into the tissue. Note that a thermocouple was inserted midway between the two electrodes.
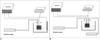
Fig. 2
Measurements of the ablated lesions: 1- overlapping width, 2- and 3- the longest diameters along the electrode, 4- the shortest diameter midway between the two electrodes.
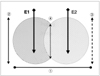
Fig. 3
Graphs of mean temperatures at the midpoint between the two electrodes in each group. Note that the highest temperatures occurred in the bipolar mode.

Fig. 4
Comparison of radiofrequency-induced coagulation created by applying radiofrequency in the three groups. Note that the mean shortest coagulation diameters midway between the two electrodes were largest in group C. Arrows indicate electrode tracks.
A. Photograph of specimen from group A
B. Photograph of specimen from group B
C. Photograph of specimen from group C

References
1. Goldberg SN, Gazelle GS, Solbiati L, Livraghi T. State of the art: tumor ablation with radio-frequency energy. Radiology. 2000. 217:633–646.
2. McGahan JP, Dodd GD. Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001. 176:3–16.
3. Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radiofrequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001. 221:159–166.
4. Lim HK. Radiofrequency thermal ablation of hepatocellular carcinomas. Korean J Radiol. 2000. 1:175–184.
5. Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radiofrequency ablation of medium and large lesions. Radiology. 2000. 214:761–768.
6. de Baere T, Elias D, Dromain C, et al. Radiofrequency ablation of 100 hepatic metastases with a mean follow-up of more than one year. AJR Am J Roentgenol. 2000. 175:1619–1625.
7. Dodd GD, Soulen MC, Kane RA, et al. Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. RadioGraphics. 2000. 20:9–27.
8. Goldberg SN, Stein M, Gazelle GS, Sheiman RG, et al. Percutaneous radiofrequency tissue ablation: optimization of pulsed-RF technique to increase coagulation necrosis. J Vasc Interv Radiol. 1999. 10:907–916.
9. Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques and diagnostic imaging guidance. AJR Am J Roentgenol. 2000. 174:323–331.
10. Lee JM, Kim YK, Lee YH, Kim SW, Li CA, Kim CS. Percutaneous radiofrequency thermal ablation with hypertonic saline injection: in-vivo study in a rabbit liver model. Korean J Radiol. 2003. 4:27–34.
11. Munver R, Threatt CB, Delvecchio FC, Preminger GM, Polascik TJ. Hypertonic saline-augmented radiofrequency ablation of VX2 tumor implanted in rabbit kidney: a short-term survival pilot study. Urology. 2002. 60:170–175.
12. Goldberg SN, Ahmed M, Gazelle GS, et al. Radiofrequency thermal ablation with NaCl solution injection: effect of electrical conductivity on tissue heating, and coagulation-phantom and porcine liver study. Radiology. 2001. 219:157–165.
13. McGahan JP, Gu WZ, Brock JM, Tesluk H, Jones CD. Hepatic ablation using bipolar radiofrequency electrocautery. Acad Radiol. 1996. 3:418–422.
14. Curley SA, Davidson BS, Fleming RY, et al. Laparoscopically guided bipolar radiofrequency ablation of areas of porcine liver. Surg Endosc. 1997. 11:729–733.
15. Goldberg SN, Hahn PF, Halpern E, et al. Radiofrequency tissue ablation: effect of pharmacologic modulation of blood flow on coagulation diameter. Radiology. 1998. 209:761–769.
16. Patterson EJ, Scudamore CH, et al. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998. 227:559–565.
17. Lee JD, Lee JM, Kim SW, Kim CS, Mun WS. MR imaging-histopathologic correlation of radiofrequency thermal ablation lesion in a rabbit liver model: observation during acute and chronic stages. Korean J Radiol. 2001. 2:151–158.
18. Motulsky H. Motulsky Harvey, editor. Comparing three or more means: analysis of variance. Intuitive Biostatistics. 1995. 1st ed. NY: Oxford University Press Inc.;155–261.
19. Burdio F, Guemes A, Burdio JM, et al. Hepatic lesion ablation with bipolar saline-enhanced radiofrequency in the audible spectrum. Acad Radiol. 1999. 6:680–686.
20. Haemmerich D, Tungjitkusolmun S, Staelin ST, Lee FT, Mahvi DM, Webster JG. Finite-element analysis of hepatic multiple probe radio-frequency ablation. IEEE Trans BioMed Eng. 2002. 49:836–842.
21. Haemmerich D, Staelin ST, Tungjitkusolmun S, Lee FT, Mahvi DM, Webster JG. Hepatic bipolar radiofrequency ablation between separated multiprong electrodes. IEEE Trans BioMed Eng. 2001. 48:1145–1152.
22. Jang IS, Rhim H, Koh BH, et al. An experimental study of simultaneous ablation with dual probes in radiofrequency thermal ablation. J Korean Radiol Soc. 2003. 48:163–169. (in Korean).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download