Abstract
Objective
To compare phase-inversion sonography during the liver-specific phase of contrast enhancement using a microbubble contrast agent with conventional B-mode sonography for the detection of VX2 liver tumors.
Materials and Methods
Twenty-three rabbits, 18 of which had VX2 liver tumor implants, received a bolus injection of 0.6 g of Levovist (200 mg/ml). During the liver-specific phase of this agent, they were evaluated using both conventional sonography and contrast-enhanced phase-inversion harmonic imaging (CE-PIHI). Following sacrifice of the animals, pathologic analysis was performed and the reference standard thus obtained. The conspicuity, size and number of the tumors before and after contrast administration, as determined by a sonographer, were compared between the two modes and with the pathologic findings.
Results
CE-PIHI demonstrated marked hepatic parenchymal enhancement in all rabbits. For VX2 tumors detected at both conventional US and CE- PIHI, conspicuity was improved by contrast-enhanced PIHI. On examination of gross specimens, 52 VX2 tumors were identified. Conventional US correctly detected 18 of the 52 (34.6%), while PIHI detected 35 (67.3%) (p < 0.05). In particular, conventional US detected only three (8.3%) of the 36 tumors less than 10 mm in diameter, but CE-PIHI detected 19 such tumors (52.8%) (p < 0.05).
The detection of hepatic metastases is a crucial issue in the care of patients with known primary cancers, and ultrasound is one of the diagnostic modalities used for this purpose (1, 2). Ultrasound is known, however, to have a relatively high false-negative rate for liver metastases (in the range of 33-47%), and its low sensitivity is related to its limited depiction of isoechogenic and small liver metastases (3-7). Levovist (SHU 508 A; Schering AG, Berlin, Germany) is an intravenously administered, galactose-based microbubble contrast agent that accumulates in normal liver parenchyma during the late liver-specific phase, but which spares focal lesions such as metastases (8-12). A number of studies have demonstrated that pulse- or phase- inversion harmonic imaging using Levovist improved the detection rate of hepatic malignancies compared with conventional ultrasound imaging (9-12). In those studies, however, the lack of firm evidence of the existence of small liver metastases was the major limitation.
The purpose of this study is to determine whether phase inversion harmonic imaging (PIHI) during the late hepatic phase of contrast enhancement with Levovist depicts a greater number of VX2 tumors, and ones that are smaller, than does conventional sonography.
All protocols were approved by the Animal Use and Care Committees of our universities. Twenty-three adult male New Zealand White rabbits each weighing 3.0-3.5 (mean, 3.2) kg were included in this study, and were allocated to either the VX2 tumor group (n=18) or the control group (n=5). VX2 tumor cells were inoculated by laparotomy into the liver of rabbits in the tumor group. Animals in the control group also underwent laparotomy, but without tumor cell inoculation, and the sonographer was thus unaware, at the time of ultrasound examination, of whether a VX2 tumor had been implanted. The rabbits were anesthetized by intramuscular injection of 50 mg/kg ketamine hydrochloride (Ketamine®; Yuhan, Seoul, Korea) and 5 mg/kg xylaine (Rumpun®; Bayer Korea, Ansan, Korea) prior to tumor inoculation and imaging studies. Booster injections of up to one-half the initial dose were administered as needed.
The VX2 tumor model was induced as already described in previous reports (13, 14). Tumor cell suspension with an estimated viable cell density of 5×106 cells/ml was prepared using a freshly harvested VX2 tumor from a carrier rabbit and inoculated via laparotomy: during a 30-second period, aliquots (0.1-0.3 ml of 5×106 viable cells/ml) of VX2 tumor cells were slowly injected into the liver at one or two locations.
Fourteen days after tumor implantation, ultrasonograms of the liver were obtained by one experienced radiologist. The abdomen was shaved, and the rabbits were placed in the supine position and attached to the investigation board. Using individually optimized settings, a Sonoline Elegra scanner (Siemens, Issaquah, Wash., U.S.A.) with a 7.5MHz linear array and a dynamic range of 55 dB was used to obtain conventional B-mode sonograms of longitudinal and transverse liver sections.
After the completion of baseline scanning, one bolus of Levovist (200 mg/mL, 3 cc) was injected into the lateral ear vein of each rabbit at 1 mL/sec using a 21-gauge scalp vein set (Green Cross Medical Co; Yongin, Kyungki, Korea). To ensure that no residual contrast agent remained in the intravenous line, 5-mL physiologic saline solution was then immediately injected. Levovist uptake is liver specific, and in order to allow sufficient time for the agent to accumulate in normal liver parenchyma, gray-scale pulse-inversion harmonic US data were recorded after a delay of between 2.5 and 5 (mean, 4) minutes. For phase-inversion scanning, "ensemble contrast imaging" software (Siemens) was used with the following settings: insonating frequency, 2.5 MHz; mechanical index, 1.0; frame rate, more than 5 per second; parallel processing; and 2-5 focal zones. The findings of a previous study (15) suggested that these mechanical index values would provide marked enhancement of the liver parenchyma and thus lead to improved tumor detection.
For phase-inversion sonography, scanning involved a controlled transverse sweep of the entire right lobe of the liver from the diaphragm to the lower pole, lasting approximately 3-4 seconds (16). When a sweep was completed, the image was frozen and the individual frames were reviewed on a cine loop without time constraints. This was followed by a transverse sweep of the left lobe using an appropriate focal zone position. After these standardized sweeps, additional phase-inversion scanning was performed as required to visualize areas of the liver that might not have already been sufficiently visualized. Representative images were stored on the hard disc of the US scanner.
The number, size, location, and echogenicity of each tumor was recorded; size was determined in order to make a lesion-to-lesion comparison between the US findings and the pathologic specimen. Echogenicity was classified as hypo-, iso- or hyperechoic, as compared with hepatic parenchyma. The findings of both baseline and phase-inversion sonography were interpreted at the time of examination, without knowledge of whether or not VX2 tumors had been implanted.
Tumor conspicuity, as seen at both baseline and PIHI, was evaluated and compared at the time of scanning, and decisions were made as to which modality provided better results. Whether each modality enabled correct identification of a rabbit with at least one implanted tumor was also determined, and recorded as "yes" or "no." In addition, the ability of each modality to depict the correct number of implanted tumors was compared. The sensitivity, specificity, and accuracy of both sonographic techniques for detecting individual VX2 tumors were calculated using the reference standard (gross pathologic analysis).
After US examination, rabbits were sacrificed by injecting an overdose of Ketamine and Xylazine, and their livers were harvested. Gross pathologic analysis of the liver provided a reference standard for comparing conventional imaging and contrast-enhanced PIHI. All livers were serially sectioned in their entirety in planes similar to those of the US examinations, and the number and size of masses were noted. The pathologic findings could thus be compared directly with the tumor numbers and locations established using conventional US and PIHI. The lesions detected were assigned to one of three groups: 10mm or more in diameter, between 5 and 9 mm, and smaller than 5 mm.
Using analysis of variance with Bonferroni post-testing, the mean numbers of reference-confirmed hepatic VX2 tumors revealed by the two sonographic techniques were compared with each other and with the number seen at reference examination (gross pathologic examination) (17). Using the paired t test, the size of the smallest VX2 tumor detectable in each rabbit was compared between each imaging technique. For statistical analysis of tumor detection rates, the McNemar test for correlated proportions was used, with p values of less than 0.05 indicating a significant difference. Because of the small number of animals, exact binomial probabilities were used in this test. The diagnostic accuracy of each imaging modality was compared by calculating both sensitivity and specificity.
On examination of gross specimens, 52 VX2 tumors were identified: 22 were less than 5 mm in diameter, 14 were between 5 and 9 mm, and 15 were 10 mm or larger. In 15 of the 18 rabbits (83.3%) in which hepatic VX2 tumors were seen at pathologic examination, conventional imaging also revealed their presence (Table 1). In 61.1% of the rabbits (11/18), more VX2 tumors were detected at PIHI with Levovist injection than at conventional US imaging (Fig. 3). Of the 52 VX2 tumors, conventional US imaging detected only 18 (34.6%), but PIHI with contrast injection detected 35 (67.3%) (p < 0.05). In addition, conventional US and CE-PIHI revealed the presence of VX2 tumors in 15 (83.3%) and 18 rabbits (100%), respectively, with positive findings. Thus, sensitivity to the presence or absence of hepatic VX2 tumor increased from 83.3% with conventional US imaging to 100% with contrast-enhanced PIHI (p > 0.05). In three rabbits with false-negative findings at conventional imaging, four tumors (two less than 5 mm in diameter, two between 5 and 9 mm) were found at pathologic examination; contrast-enhanced PIHI detected three of four nodules (Fig. 2). Furthermore, contrast-enhanced PIHI detected significantly smaller VX2 tumors than did conventional US imaging (p < 0.05): the latter depicted 19 of 36 tumors less than 10 mm in diameter (52.8%), while the former revealed only three such tumors (8.3%) (Table 1).
Conventional US imaging showed that tumors were characterized by isoechoic or slightly hyperechoic tissues; because of signs of compression of surrounding liver parenchyma and the formation of central necrosis and cyst-like structures, those larger than 5 mm were, however, only partially visible (Fig. 1). The use of contrast-enhanced PIHI led to marked hepatic parenchymal enhancement, i.e. markedly increased liver echogenicity. Tumors were clearly delineated as areas devoid of enhancement that appeared as hypo- or nearly anechoic lesions (Fig. 1). The conspicuity of tumors detected at both conventional US and CE-PIHI was greater at CE-PIHI (Figs. 1 and 2).
Data analysis showed that conventional imaging permitted the correct identification of 18 of 52 tumors and all five normal control animals. and its sensitivity and specificity were thus 34.6% and 100%, respectively. Because 34 false-negative results were obtained at conventional US, the difference between this modality and the pathologic findings was significant (p < 0.001). At CE-PIHI, the number of false negatives was reduced to 17. The contrast-enhanced US findings were, however, significantly different from those of pathology (p < 0.05): following the administration of Levovist, sensitivity increased to 67%, and there was thus significant difference between the sensitivity of US with and without contrast agent (p < 0 .05).
With the use of increasingly aggressive surgical techniques for the management of liver metastases, the task of preoperative imaging has become more demanding. To this end, a variety of imaging modalities, including US, computed tomography (CT) and magnetic resonance imaging( MRI), have been used for the detection of hepatic metastases (18,19), and in recent years, as contrast-specific imaging modes such as wide-band harmonic imaging and stimulated acoustic emission have developed, the use of various sonographic contrast agents has gained popularity (8-12, 20-24). Contrast-specific ultrasound imaging techniques are highly sensitive to both microbubble movement and microbubble collapse, independently of the level of applied acoustic peak pressure. When increasing pressure (high mechanical index), the microbubbles are destroyed, leading to strong acoustic enhancement of the tissue containing them (8-12). Compared to color Doppler harmonic US, PIHI has a number of features advantageous for parenchymal imaging: higher spatial and temporal resolution, and less motion artifact and contrast agent-induced blooming (20-22). Previous studies (11,12,15,16) have indicated that this strong acoustic enhancement of liver parenchyma induced by PIHI during the late phase of Levovist leads to improved detection of small liver tumors by improving contrast between the tumors and liver parenchyma (11,12,16). A limitation of those studies, however, was their lack of definitive pathologic proof.
Contrast-enhanced PIHI proved particularly useful for detecting small VX2 tumors (less than 10 mm in diameter) that were often invisible on baseline images but became apparent due to the marked increase in contrast between a nonenhancing lesion and enhancing surrounding parenchyma. Conventional imaging detected only three (8.3%) of 36 tumors less than 10 mm in diameter, while contrast-enhanced PIHI demonstrated 16 additional nodules (52.8%; 19/36) (p < 0.05). PIHI showed that during the late hepatic phase of contrast enhancement with Levovist, hepatic parenchymal enhancement was homogeneous in all rabbits, but hepatic VX2 tumors were clearly delineated as areas devoid of enhancement. Contrast between tumors and liver parenchyma thus showed remarkable improvement at contrast-enhanced PIHI compared to conventional US imaging, suggesting that PIHI can increase the detectability of small VX2 tumors. Although it is not yet clear precisely where Levovist accumulates inside the liver, there is most likely some form of non-phagocytic interaction with cells of the reticuloendothelial system (11, 12, 24). The low rate of detection of small lesions, one of the main limitations of conventional sonography, was, therefore, overcome with contrast-enhanced PIHI. Although it is still possible that tumors less than 5 mm in diameter might not be detectable in cirrhotic human liver, nor do CT and MR imaging detect small tumors at rates which are satisfactorily high (4,6, 25). The results of the present study with Levovist are, therefore, encouraging.
However, even when PIHI and a contrast agent were used, the detection rate of tumors smaller than 5 mm in diameter was only 27.3% (6/22). In addition, contrast-enhanced PIHI revealed, in total, only 67.3% of hepatic VX2 tumors, a relatively poor result compared to that of a previous study using a different US contrast medium (20). Forsberg et al. (14) stated that pulse-inversion harmonic imaging using NC 100100 (Sonazoid; Amersham Health, Oslo, Norway) detected 93% of hepatic VX2 tumors. Although we cannot explain why our results were worse than in that earlier study, the low detection rate associated with contrast-enhanced US for VX2 tumors may be related to the transient nature of late-phase enhancement with Levovist. Using the current high-mechanical-index PIHI technique, only part of the liver can be visualized in one or two sweeps because of rapid bubble depletion. A potential solution to this problem may be the use of recent low-mechanical-index techniques allowing several sweeps to be performed using the same bubble population, or slow contrast infusions with continuous bubble replenishment (16).
The major limitation of this study was that the lesion-to-lesion comparison at unenhanced conventional US and contrast-enhanced PIHI was sometimes difficult because of the limited imaging planes used for PIHI during sweeping. When correlating individual lesions, some degree of flexibility was therefore required. When it was difficult to match individual lesions, and both US examination modes showed that their size and distribution were similar, we assumed that they were identical. This approach is certainly susceptible to error but has no obvious remedy.
This study demonstrated that phase-inversion sonography during the late phase of contrast enhancement with Levovist detects more hepatic VX2 tumors than conventional sonography. We therefore believe that the use of CE-PIHI can improve the detection of small human liver tumors and that for liver imaging, this modality may become a competitive alternative to modalities such as CT and MR imaging. To compare the diagnostic performance of PIHI with that of other imaging modalities, further study is warranted.
Figures and Tables
Fig. 1
A. Unenhanced baseline transverse conventional US image obtained in a rabbit shows a 10 mm, slightly hyperechoic hepatic VX2 tumor (arrows).
B. Transverse phase-inversion harmonic US image obtained in the same animal five minutes after contrast injection shows homogeneous enhancement of normal liver parenchyma. Note the hepatic VX2 tumor, seen as a well-defined hypoechoic enhancement defect (arrows).
C. Transverse section of a gross specimen reveals an oval-shaped, whitish VX2 liver tumor (arrows).
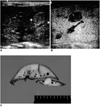
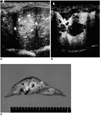
Fig. 2
A. Unenhanced subcostal transverse sonogram of rabbit liver depicts a heterogeneous isoechoic lesion (arrows).
B. Contrast-enhanced transverse phase-inversion US image obtained slightly inferior to the unenhanced image (A) reveals three nonenhancing foci measuring 2-12 mm in diameter. Two additional lesions (arrows) are identified, and their conspicuity is much greater than at unenhanced conventional imaging (A).
C. Transverse section of the liver reveals five tumors at the same location. Compared to B, two additional lesions (arrows) less than 5 mm in diameter are apparent.
Acknowledgments
The authors wish to thank Seon Ok Lee, R.T., Department of Radiology, Chonbuk National University Hospital, for her assistance in animal observation and anesthesia. We also wish to acknowledge the editorial assistance of Bonnie Hami, M.A., Department of Radiology, University Hospitals of Cleveland.
References
1. Bluemke DA, Paulson EK, Choti MA, DeSena S, Calvien PA. Detection of hepatic lesions in candidates for surgery. AJR Am J Roentgenol. 2000. 175:1653–1658.
2. Alderson PO, Adams DF, McNeil BJ, et al. Computed tomography, ultrasound, and scintigraphy of the liver in patients with colon or breast carcinoma: a prospective comparison. Radiology. 1983. 149:225–230.
3. Soyer PH, Levesque M, Elias D, Zeitoun G, Roche A. Detection of liver metastases from colorectal cancer: comparison of intraoperative ultrasound and CT during arterial portography. Radiology. 1992. 183:541–544.
4. Wernecke K, Rummeny E, Bongartz G, et al. Detection of hepatic masses in patients with carcinoma: comparative sensitivities of sonography, CT, and MR imaging. AJR Am J Roentgenol. 1991. 157:731–739.
5. Clarke MP, Kane RA, Steele G Jr, et al. Prospective comparison of preoperative imaging and intraoperative ultrasonography in the detection of liver tumors. Surgery. 1989. 106:849–855.
6. Ohlsson B, Tranberg KG, Lundstedt C, Ekberg H, Hederstrom E. Detection of hepatic metastases in colorectal cancer: a prospective study of laboratory and imaging methods. Eur J Surg. 1993. 159:275–281.
7. Ferrucci JT. Liver tumor imaging: current concepts. AJR Am J Roentgenol. 1990. 155:473–484.
8. Burns PN, Wilson SR, Simpson DH. Pulse-inversion imaging of liver blood flow: improved method for characterizing focal masses with microbubble contrast. Invest Radiol. 2000. 35:58–71.
9. Wilson SR, Burns PN, Muradali D, Wilson JA, Lai X. Harmonic hepatic US with microbubble contrast agent: initial experience showing improved characterization of hemangioma, hepatocellular carcinoma, and metastases. Radiology. 2000. 215:153–161.
10. Blomley MJK, Albrecht T, Cosgrove D, Jayaram V, Butler-Barnes J, Eckersley R. Stimulated acoustic emission in liver parenchyma with Levovist (letter). Lancet. 1998. 351:568.
11. Blomley MJK, Albrecht T, Cosgrove DO, et al. The use of stimulated acoustic emission to image a late liver-specific phase of Levovist: an investigation in normal volunteers and in patients with and without liver disease. Ultrasound Med Biol. 1999. 25:1341–1352.
12. Blomley MJK, Albrecht T, Cosgrove DO, et al. Improved imaging of liver metastases with stimulated acoustic emission in the late phase of enhancement with the US contrast agent SHU 508A: early experience. Radiology. 1999. 210:409–416.
13. Forsberg F, Liu JB, Merton DA, Rawool NM, Johnson DK, Goldberg BB. Gray-scale second harmonic imaging of acoustic emission signals improves detection of liver tumors in rabbits. J Ultrasound Med. 2000. 19:557–563.
14. Forsberg F, Piccoli CW, Rawool NM, Merton DA, Mitchell DG, Goldberg BB. Hepatic tumor detection: MR imaging and conventional US versus pulse-inversion harmonic US of NC100100 during its reticuloendothelial system-specific phase. Radiology. 2002. 222:824–829.
15. Albrecht T, Hoffmann CW, Schettler S, et al. B-mode enhancement at phase-inversion US with air-based microbubble contrast agent: initial experience in humans. Radiology. 2000. 216:273–278.
16. Albrecht T, Hoffmann CW, Schmitz SA, et al. Phase-inversion sonography during the liver-specific late phase of contrast enhancement: improved detection of liver metastases. AJR Am J Roentgenol. 2001. 176:1191–1198.
17. Lang TM, Secic M. How to report statistics in medicine: annotated guidelines for authors, editors, and reviewers. 2000. 1st ed. Philadelphia: ACP;127–132.
18. Finlay IG, McArdle CS. Effect of occult hepatic metastases on survival after curative resection for colorectal carcinoma. Gastroenterology. 1983. 85:596–599.
19. Hughes KS, Sugarbaker PH. Rosenberg SA, editor. Treatment of metastatic liver tumors. Surgical cure of metastatic cancer. 1987. Philadelphia: Lippincott;125–164.
20. Bauer A, Schlief R, Zomack M, Urbank A, Niendorf HP. Nanda N, Schlief R, Goldberg BB, editors. Acoustically stimulated microbubbles in diagnostic ultrasound: properties and implications for diagnostic use. Advances in echo imaging using contrast enhancement. 1997. 2nd ed. London: Kluwer;669–684.
21. Simpson DH, Chin CT, Burns PN. Pulse-inversion Doppler: a new method for detecting nonlinear echoes from microbubble contrast agents. IEEE Trans Ultrason Ferroelectr Freq Control. 1999. 46:372–382.
22. Forsberg F, Liu JB, Chiou HJ, Rawool NM, Parker L, Goldberg BB. Comparison of fundamental and wideband harmonic contrast imaging of liver tumors. Ultrasonics. 2000. 38:110–113.
23. Simpson DH, Burns PN. Pulse-inversion Doppler: a new method for detecting nonlinear echoes from microbubble contrast agents. IEEE Ultrasonic Symposium. 1997. 1597–1600.
24. Quaia E, Blomley MJK, Patel S, et al. Initial observations on the effect of irradiation on the liver-specific uptake of Levovist. Eur J Radiol. 2002. 41:192–199.
25. Mitsuzaki K, Yamashita Y, Ogata I, et al. Multiple-phase helical CT of the liver for detecting small hepatomas in patients with liver cirrhosis: contrast-injection protocol and optimal timing. AJR Am J Roentgenol. 1996. 167:753–757.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download