Abstract
Objective
To determine the value of selective intra-arterial calcium stimulation with hepatic venous sampling using serum insulin and C-peptide gradients for the preoperative localization of insulinomas.
Materials and Methods
Seven consecutive patients [three men and four women aged 15-77 (mean, 42.7) years] with hypoglycemia underwent selective intra-arterial calcium stimulation in conjunction with hepatic venous sampling. Insulin gradients were calculated by an individual blinded to all other preoperative imaging studies and operative findings. In all patients except one, C-peptide gradients were also analyzed. The results were compared with the preoperative findings of ultrasonography, computed tomography, arteriography and endoscopic ultrasonography, as well as with the intraoperative findings of ultrasonography and palpation at surgery.
Results
Eight insulinomas (mean diameter, 12.5 mm) were diagnosed after surgery. In six patients, the calcium stimulation test with insulin gradients allowed accurate localization of the pathologic source of insulin secretion. Both C-peptide and insulin gradients substantially increased diagnostic accuracy. In one patient, C-peptide gradients were more helpful than insulin gradients for tumor localization.
Pancreatic insulinoma is a relatively rare endocrine tumor with an estimated occurrence rate of 4 cases per million person-years (1). Thus, few clinicians or even referral centers have extensive experience of the condition. Most insulinomas are small, and 90% are smaller than 2 cm (2). Although 90% of these tumors are benign, they are still potentially fatal due to the risk of lethal hypoglycemia (3). If patients successfully undergo curative surgical resection (4, 5), they have a normal life expectancy (1).
Insulinoma can be confirmed by demonstrating that during hypoglycemia the plasma insulin level is inappropriately high, and modern suppression tests based upon this fact allow verification with a high degree of confidence (6). Preoperative localization of an insulinoma is a crucially important step after diagnosis, and leads to significant reductions in operative time (2).
In 1991, Doppman et al. (7) reported four cases in which insulinomas were successfully localized using 0.01-0.025 mEq/kg calcium gluconate as the secretagogue. In 1993, authors from the same institution reported the successful localization of six additional tumors (8). Other recent studies have shown that selective intra-arterial stimulation with hepatic venous sampling using intra-arterial calcium as the insulin secretagogue is a powerful technique for the localization of occult insulinomas (4, 7-12). Doppman et al. (8) found, however, that elevated hepatic venous insulin levels did not always equate with correct localization: unequivocal localization was reported in only five of nine patients (56%) but a gradient was demonstrated in all nine. Thus, we supposed that additional analysis of C-peptide gradients would be helpful in assessing the location of a tumor when insulin gradients are equivocal.
The purpose of our study was to demonstrate the value of selective intra-arterial calcium stimulation with hepatic venous sampling (ASVS), using serum C-peptide and insulin gradients for the localization of insulinomas. The results of ASVS were compared with the preoperative findings of US, CT, angiography, and endoscopic US (EUS), as well as with the findings of intraoperative US (IOUS) and palpation at surgery.
Seven consecutive patients [three men and four women aged 15-77 (mean, 42.7) years] admitted to our hospital with hypoglycemia between April 1, 2001 and September 31, 2002 were retrospectively studied. The time from the onset of neuroglycopenic symptoms to diagnosis ranged from 4 to 42 months. The age, sex, and history of the patients are summarized in Table 1. The inclusion criteria were coexisting hypoglycemic symptoms with low fasting serum glucose levels (<50 mg/dL) and biochemical evidence of organic hyperinsulinemia, confirmed by a fasting test (serum insulin:glucose ratio (0.3, with high C-peptide levels). Symptomatic hypoglycemia was observed and documented [plasma glucose range: 27-46 (mean, 35) mg/dl] after 24-72 hours of fasting. Concomitant insulin [range: 10.9-33.6 (mean, 18.4) µIU/ml] and C-peptide levels [range: 1.95-5.67 (mean, 3.95) ng/ml] were not suppressed. All these patients had pathologic glucose-insulin ratios (range, 0.40-0.73; mean, 0.51). Thus, hyperinsulinemic hypoglycemia due to insulin-secreting tumors was proven in all cases. Fasting test results are summarized in Table 1.
Radiological imaging modalities employed for the localization of these pancreatic adenomas are summarized in Table 2. Ultrasonography (US) was performed in three cases, endoscopic ultrasonography (EUS) in two, computed tomography (CT) in seven, and intraoperative ultrasonography (IOUS) in four. In all cases, pancreatic angiography formed part of the calcium stimulation test. The detection rate of each study was evaluated.
After obtaining informed consent from each patient, ASVS was performed according to the protocol proposed by Doppmann et al. (8). Additional C-peptide sampling had also been consented to. None of our patients was receiving cardiac glycosides, and none was suffering from sarcoidosis or renal failure, conditions considered relative contraindications to intra-arterial calcium injection. All tests were carried out under local anesthesia and with continuous electrocardiographic and blood pressure monitoring. To avoid iatrogenic hypoglycemia, blood sugar levels were checked before the procedure. In patient 2, this was 37 mg/dl, and 25 g of intravenous glucose were administered, using a 50% solution.
Prior to angiography, a 5-French (F) Cobra catheter (Terumo, Tokyo, Japan) was placed in the right hepatic vein close to its junction with the inferior vena cava through a puncture site in the right internal jugular vein (Fig. 1). The left hepatic vein was not catheterized. The right femoral artery was then punctured, and a 5-F Yashiro catheter (Terumo) was advanced to the celiac trunk and the superior mesenteric artery (SMA). Occasionally, a 3-F Progreat microcatheter (Terumo) was used for coaxial superselective catheterization of the gastroduodenal (GDA) and splenic artery (SpA). Selective presampling angiography was performed after selectively injecting nonionic contrast agent (Iomeron 300; Ilsung, Korea) into the GDA, SpA, and SMA. For angiographic studies, digital subtraction angiography (DSA) was used (Fig. 1).
Immediately after each such study, a 5-ml bolus of calcium gluconate (0.025 mEq Ca2+/kg) diluted in saline was rapidly injected through the proximally positioned catheter in each selectively catheterized artery. The period between each injection was at least 5 minutes. Before and 30, 60, and 120 seconds after calcium injection, 5 ml of hepatic venous blood was obtained. Each sample was labeled according to the time interval and artery of origin, and centrifuged, and the resulting serum was assayed for insulin concentration in all patients and C-peptide concentration in six.
The accuracy of ASVS in localizing pancreatic insulinomas was retrospectively determined. All ASVS results were analyzed by an individual blinded to all other preoperative imaging studies and operative findings. According to the findings of Doppman et al., a positive finding is indicated by a twofold elevation of insulin levels in the 30- or 60-second samples (or both) obtained from the hepatic vein (1). A twofold rise in insulin levels following calcium gluconate injection of the GDA or SMA indicates that the tumor is located in the pancreatic head or uncinate process; a twofold rise after injection into the SpA suggests a corporeal or caudal location. For diagnostic accuracy, C-peptide gradients in all patients except patient 1 were also analyzed. The diagnostic criteria for C-peptide have not been reported, but we supposed that such analysis would be useful if insulin gradients were equivocal. The additional utility of C-peptide gradients will be discussed later.
The results of ASVS in which insulin gradients only were determined, and in which additional C-peptide gradients were established, were compared. Comparisons were also made between the sensitivity of these modalities and that of other localizing modalities in terms of the operative findings and outcome of surgery.
All patients later underwent surgery, preferably enucleation of the adenoma. Routine bimanual palpation was applied in all cases, and IOUS in four. The ASVS results were compared with the operative findings and the outcome of surgery.
The median follow-up time was 4 (range, 1-10) months. At these intervals, patient histories were obtained and plasma glucose levels were determined after 24-72 hours of fasting.
The results of all localization studies and surgery are presented in Table 2.
In no case did ASVS lead to complications, and procedural-related symptomatic hypoglycemia and hypercalcemia were not encountered. Insulin or C-peptide gradients were calculated as the ratios of hepatic venous insulin or C-peptide concentrations, respectively, at 30, 60, and 120 seconds after ASVS to baseline.
In patients 1, 2, 5 and 7, significant insulin gradients were observed after calcium injection into the GDA and in patient 6 after stimulation of the GDA and SMA, indicating the presence of insulinomas in the pancreatic head or uncinate process (Fig. 2A). In patients 2, 5, 6 and 7, C-peptide gradients were also significant (Fig. 2B), and in patient 3, both insulin and C-peptide gradients increased significantly after stimulation of the SpA, suggesting that the pathological source of insulin might be the pancreatic body or tail (Fig. 3).
A diagnostic problem occurred only in patient number 4. Peak insulin gradients in the GDA 30 seconds after stimulation and in the SpA at 60 seconds were not significantly different (Fig. 4A). Moreover, after 30 seconds, insulin gradients decreased gradually in the GDA, but in the SpA showed a gradual increase. The analyst, blinded to all other preoperative imaging studies and operative findings, thus could not confidently determine the location of the insulinoma. In this case, additional C-peptide gradients were, however, helpful (Fig. 4B). The C-peptide gradient peaked 60 seconds after stimulation of the SpA, and in the GDA was not more than 1.0. The analyst finally determined that the tumor location was the pancreatic body or tail.
Eight insulinomas, 5-25 (mean, 12.5) mm in size, were found and confirmed histopathologically after enucleation (Table 2). All tumors except in patient 3 were less than 2 cm in size. Three were in the head, two in the uncinate process and three in the tail. In patient 4, two small adenomas were detected in the pancreatic tail.
The tumors were correctly localized by US in two of three patients (sensitivity, 67%), by EUS in one of two (sensitivity, 50%), by CT in four of seven (sensitivity, 57%), by angiography in three of seven (sensitivity, 43%), and by IOUS in four of four (sensitivity, 100%).
For insulin gradients only, the sensitivity of ASVS was 86% (6 of 7 patients), but with additional C-peptide gradients, sensitivity was 100% (6 of 6 patients).
All patients were cured by enucleation of the insulinoma and remained free of hypoglycemia during the follow-up period.
The availability of methods to accurately determine circulating levels of insulin, C-peptide, and proinsulin has led to dramatic improvements in the preoperative diagnosis of insulinomas, and to exclusion of the surreptitious administration of insulin as the cause of hypoglycemia (3). Preoperative localization of a tumor has, for this reason, moved to center stage as the main focus of interest.
Since Daggett et al. (6) published their findings in 1981, the question of whether imaging is necessary prior to initial insulinoma-related surgery has been a matter of debate (13-15). Many surgical teams, meanwhile, do not rely exclusively on bimanual palpation of the pancreas combined with IOUS, but insist on meticulous preoperative localization, and it is to this that they attribute their high operative success rates (16-18). An average of 10% of insulinomas defy surgical exploration (4, 16) and correct preoperative localization minimizes the extent to which this is necessary, thus reducing morbidity (13). Recent developments in the laparoscopic resection of pancreatic insulinomas, a procedure increasingly described for tumors located in the body or tail of the pancreas, emphasize, furthermore, the need for an accurate preoperative localization procedure (19, 20). Hence, even though they are controversial in terms of cost-effectiveness (3, 22), preoperative localization studies are recommended (13, 15-17, 21). If such studies do not facilitate rational and safe surgical enucleation or resection, they are not, however, time- and cost-effective (23).
The mean diameter of insulinomas is generally not more than 15 mm (2, 11, 15, 24), and non-invasive imaging modalities thus often fail to localize them. Non-invasive modalities such as US, CT, and MRI have shown low sensitivities (8, 9, 13, 14, 16, 21, 25), while CT and US will detect up to 60% of biochemically proven insulinomas. Relatively little has so far been reported about MRI in the diagnosis of islet cell tumors, and its exact role is uncertain (4). In our series the mean size of insulinomas was 12.5 mm, a fact which could explain the low detection rate of adenomas at US and CT. However, recent reports describing the findings of dynamic CT and MRI have been promising (26-28).
The sensitivities of other, invasive, localization techniques for detecting insulinomas were higher: 57-82% with EUS (11, 12, 18, 29, 30), 35% to 65% with angiography (9, 10, 31, 32), and 77-100% with transhepatic portal venous sampling (TPVS) (7-10, 13, 14, 17, 21, 31, 32). However, these techniques have certain disadvantages, including low sensitivity in the case of angiography and EUS (the sensitivity of EUS depends on the location of the tumor) and higher morbidity for TPVS (4, 8, 9, 33).
The sensitivity of IOUS combined with palpation was more than 80% in most reported series (2-4, 14, 16, 31, 34). However, insulinomas located in the pancreatic head or uncinate process, and thus deep within the parenchyma, were difficult or impossible to palpate, even where an experienced surgeon was involved (5), and to detect at surgery, using IOUS, particularly at hospitals with limited experience of this modality (9, 11, 31).
With ASVS, as introduced by Doppman et al. in 1991 (3, 7), the detection of insulinomas is based on their hormone production, not their appearance. The sensitivity of the technique is independent of lesion size. At the same time, however, since the lesion is identified by its hormone output, it is not visulalized directly and cannot be located precisely. Its position can be confirmed only in terms of a region of the pancreas (head/uncinate process versus body/tail). Decisions as to the method of treatment are not, however, problematic. Adenomas to the left of the SMA can be treated by enucleation and distal pancreatectomy, whereas those to the right require enucleation. In our series, enucleation was preferred. Moreover, ASVS is far less invasive and complicated than TPVS and can be safely carried out in combination with a normal DSA procedure (5, 8, 9).
Complications of ASVS are rare (33), and major complications did not occur in our patients. Although hypoglycemia is a severe, even life-threatening potential complication of ASVS, it has not previously been reported (4). Insulin levels in the hepatic vein usually peaked in samples obtained 30 or 60 seconds after calcium stimulation, and were often returning toward baseline within 120 seconds of stimulation, suggesting that insulin release is confined to the short interval during which beta cells are exposed to high serum calcium levels (9). Calcium should be administered cautiously to patients with sarcoidosis, or renal or cardiac disease, particularly to those receiving cardiac glycosides (7). The inotropic effects of cardiac glycosides and calcium are synergistic; if both are administered simultaneously, dysrhythmia may thus occur (33). The injection of calcium can also lead to pancreatitis (8, 33).
A diagnostic problem occurred only in patient 4. Insulin gradients in the GDA 30 seconds after stimulation and in the SpA at 60 seconds were not significantly different, and while this gradient peaked 30 seconds after the start of calcium gluconate infusion into the GDA, in the SpA it increased gradually. Additional analysis of the C-peptide gradient showed that in the SpA it peaked 60 seconds after stimulation, and in the GDA it was not more than 1.0. The analyst localized the tumor to the pancreatic body or tail, and two small adenomas found in the tail were to the left of the SMA.
Elevated insulin levels in the hepatic veins after ASVS did not always equate with correct localization (8). We assumed that additional analysis of C-peptide gradients would help assess the location of a tumor when insulin gradients are equivocal, basing our assumption on two factors: first, calcium gluconate administration led not only to hyperinsulinemia, but also to increased serum proinsulin and C-peptide levels (35). Second, although the secretory ratio of C-peptide to insulin is 1:1, the ratio in serum is about 5:1 to 15:1 (36). The molar concentration of C-peptide in blood is higher than that of insulin because of the hepatic clearance of the latter (36). Approximately 50% of insulin is rapidly removed by its initial passage through the liver, but hepatic extraction of C-peptide is negligible (36). A relatively high concentration of C-peptide may thus reflect a subtle increase in its level, compared to that of insulin, after stimulation with calcium gluconate.
In six of our patients, the sensitivity of C-peptide gradients for localizing insulinoma was 100%. To our knowledge, this is the first report to describe the use of these gradients in ASVS for the localization of insulinomas. However, to confirm the efficacy of C-peptide gradients, in spite of the significant additional cost of multiple C-peptide assays, further investigation is needed.
A further point is that because catheterization of the left hepatic vein was technically more difficult, and also unnecessary, the right hepatic vein only was chosen (4, 8, 9, 19, 24). Thus, the amount of sampling required was also reduced. Selective distribution of splenic and mesenteric venous blood to the left and right portal vein, respectively, may mask an adenoma in the body-tail region, but such portal flow dynamics have never been proven (24). Moreover, Lo et al. (19) reported that where ASVS was used, there was no difference in the degree of step-up between right and left hepatic vein sampling. Doppman et al., furthermore, observed that in patients with tumors in the body and tail of the pancreas, insulin levels in samples obtained from the right hepatic vein were always elevated (9).
In conclusion, ASVS is a highly accurate and safe method for the preoperative localization of insulinomas. Additional C-peptide gradients seem to be helpful in assessing the location of a tumor, but additional investigation is needed.
Figures and Tables
Fig. 1
A 5-French Cobra catheter (arrow) was placed in the right hepatic vein close to its junction with the inferior vena cava through a puncture of the right internal jugular vein. Selective splenic arteriogram shows no abnormal staining.

Fig. 2
A 59-year-old woman with an insulinoma of the pancreatic head (patient 2).
A. Selective intra-arterial calcium injection demonstrates a 21-fold insulin gradient in the gastroduodenal artery at 30 secs.
B. In the same artery, a six-fold C-peptide gradient was also noted at 30 secs.
C. Gastroduodenal arteriogram demonstrates tumor blush (arrow) within the pancreatic head.
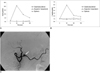
Fig. 3
A 15-year-old youth (patient 3) with an insulinoma of the pancreatic tail.
A. In the splenic artery, the insulin gradient peaked at 30 secs.
B. In the same artery, the C-peptide gradient also peaked at 30 secs.
C. Splenic arteriogram shows tumor staining (arrow) within the pancreatic tail.

Fig. 4
A 19-year-old male (patient 4) with two insulinomas of the pancreatic tail.
A. Insulin gradients show no significant difference between the GDA 30 secs after calcium stimulation and the SpA at 60 secs. Moreover, after 30 secs, insulin in the gastroduodenal artery decreased gradually, but in the splenic artery a gradual increase was observed. The analyst could not determine the location of the insulinomas.
B. In the splenic artery, the C-peptide gradient peaked 60 secs after stimulation but in the gastroduodenal artery, the gradient was not more than 1.0. The analyst localized the tumor to the pancreatic body or tail.

References
1. Service FJ, McMahon MM, O'Brien PC, Ballard DJ. Functioning insulinoma-incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc. 1991. 66:711–719.
2. Chatziioannou A, Kehagias D, Mourikis D, et al. Imaging and localization of pancreatic insulinomas. Clin Imaging. 2001. 25:275–283.
3. Axelrod L. Insulinoma: Cost-effective care in patients with a rare disease. Ann Intern Med. 1995. 123:311–312.
4. O'Shea D, Rohrer-Theurs AW, Lynn JA, Jackson JE, Bloom SR. Localization of insulinomas by selective intraarterial calcium injection. J Clin Endocrinol Metab. 1996. 81:1623–1627.
5. Chavan A, Kirchhoff TD, Brabant G, Scheumann GF, Wagner S, Galanski M. Role of the intra-arterial calcium stimulation test in the preoperative localization of insulinomas. Eur Radiol. 2000. 10:1582–1586.
6. Daggett PR, Goodburn EA, Kurtz AB, et al. Is preoperative localisation of insulinomas necessary? Lancet. 1981. 1:483–486.
7. Doppman JL, Miller DL, Chang R, Shawker TH, Gorden P, Norton JA. Insulinomas: localization with selective intraarterial injection of calcium. Radiology. 1991. 178:237–241.
8. Doppman JL, Miller DL, Chang R, Gorden P, Eastman RC, Norton JA. Intraarterial calcium stimulation test for detection of insulinomas. World J Surg. 1993. 17:439–443.
9. Doppman JL, Chang R, Fraker DL, et al. Localization of insulinomas to regions of the pancreas by intra-arterial stimulation with calcium. Ann Intern Med. 1995. 123:269–273.
10. Tsagarakis S, Kaskarelis J, Malagari C, et al. Regionalization of occult pancreatic insulinomas with the arterial stimulation venous sampling (ASVS) technique. Clin Endocrinol (Oxf). 1997. 47:753–757.
11. Pereira PL, Roche AJ, Maier GW, et al. Insulinoma and islet cell hyperplasia: value of the calcium intraarterial stimulation test when findings of other preoperative studies are negative. Radiology. 1998. 206:703–709.
12. Kuzin NM, Egorov AV, Kondrashin SA, Lotov AN, Kuznetzov NS, Majorova JB. Preoperative and intraoperative topographic diagnosis of insulinomas. World J Surg. 1998. 22:593–598.
13. Bottger TC, Junginger T. Is preoperative radiographic localization of islet cell tumors in patients with insulinoma necessary? World J Surg. 1993. 17:427–432.
14. Rothmund M. Localization of endocrine pancreatic tumours. Br J Surg. 1994. 81:164–166.
15. van Heerden JA, Grant CS, Czako PF, Service FJ, Charboneau JW. Occult functioning insulinomas: which localizing studies are indicated? Surgery. 1992. 112:1010–1015.
16. Norton JA, Shawker TH, Doppman JL, et al. Localization and surgical treatment of occult insulinomas. Ann Surg. 1990. 212:615–620.
17. Pasieka JL, McLeod MK, Thompson NW, Burney RE. Surgical approach to insulinomas: Assessing the need for preoperative localization. Arch Surg. 1992. 127:442–447.
18. Rösch T, Lightdale CJ, Botet JF, et al. Localization of pancreatic endocrine tumors by endoscopic ultrasonography. N Engl J Med. 1992. 326:1721–1726.
19. Lo CY, Chan FL, Tam SC, Cheng PW, Fan ST, Lam KS. Value of intra-arterial calcium stimulated venous sampling for regionalization of pancreatic insulinomas. Surgery. 2000. 128:903–909.
20. Gagner M, Pomp A, Herrera MF. Early experience with laparoscopic resections of islet cell tumors. Surgery. 1996. 120:1051–1054.
21. Vinik AI, Delbridge L, Moattari R, Cho K, Thompson N. Transhepatic portal vein catheterization for localization of insulinomas: a ten-year experience. Surgery. 1991. 109:1–11.
22. Rothmund M, Angelini L, Brunt LM, et al. Surgery for benign insulinoma: an international review. World J Surg. 1990. 14:393–399.
23. Norton JA. Invited commentary on pre- and intraoperative localization of insulinomas. World J Surg. 1988. 12:396–397.
24. Defreyne L, Konig K, Lerch MM, et al. Modified intra-arterial calcium stimulation with venous sampling test for preoperative localization of insulinomas. Abdom Imaging. 1998. 23:322–331.
25. Doppman JL, Shawker TH, Miller DL. Localization of islet cell tumors. Gastroenterol Clin North Am. 1989. 18:793–804.
26. Semelka RC, Cumming MJ, Shoenut JP, et al. Islet cell tumors: Comparison of dynamic contrast-enhanced CT and MR imaging with dynamic gadolinium enhancement and fat suppression. Radiology. 1993. 186:799–802.
27. Mori M, Fukuda T, Nagayoshi K, et al. Insulinoma: correlation of short-T1 inversion-recovery (STIR) imaging and histopathologic findings. Abdom Imaging. 1996. 21:337–341.
28. Kraus BB, Ros PR. Insulinoma: Diagnosis with fat-suppressed MR imaging. AJR Am J Roentgenol. 1994. 162:69–70.
29. Schumacher B, Lubke HJ, Frieling T, Strohmeyer G, Starke AA. Prospective study on the detection of insulinomas by endoscopic ultrasonography. Endoscopy. 1996. 28:273–276.
30. Thompson NW, Czako PF, Fritts LL, et al. Role of endoscopic ultrasonography in the localization of insulinomas and gastrinomas. Surgery. 1994. 116:1131–1138.
31. Doherty GM, Doppman JL, Shawker TH, et al. Results of a prospective strategy to diagnose, localize, and resect insulinomas. Surgery. 1991. 110:989–997.
32. Böttger TC, Weber W, Beyer J, Junginger T. Value of tumor localization in patients with insulinoma. World J Surg. 1990. 14:107–114.
33. Baba Y, Miyazono N, Nakajo M, Kanetsuki I, Nishi H, Inoue H. Localization of insulinomas. Comparison of conventional arterial stimulation with venous sampling (ASVS) and superselective ASVS. Acta Radiol. 2000. 41:172–177.
34. Galiber AK, Reading CC, Charboneau JW, et al. Localization of pancreatic insulinoma: Comparison of pre- and intraoperative US with CT and angiography. Radiology. 1988. 166:405–408.
35. Kaplan EL, Rubenstein AH, Evans R, Lee CH, Klementschitsch P. Calcium infusion: a new provocative test for insulinomas. Ann Surg. 1979. 190:501–507.
36. Knudson PE, Weinstock RS, Henry JB. Henry JB, editor. Carbohydrates. Clinical Diagnosis and Management by Laboratory Methods. 2001. 20th ed. Philadelphia: Saunders;211–223.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download