Abstract
Objective
To determine the heating characteristics of needle-shaped duplex stainless steel thermoseeds, and to evaluate their effectiveness in the induction of hyperthermia in rabbit liver.
Materials and Methods
Thermoseeds of the two different shapes, L-shaped for single doses of hyperthermia and I-shaped for in-vitro study and repeated hyperthermic induction, were prepared. For the in-vitro study, an I-shaped thermoseed 0.23 mm in diameter and 25 mm long was placed inside a plastic tube filled with water. Heat was applied for 30 minutes within an induction magnetic field, and during this time changes in temperature were recorded using three thermocouples. For the in-vivo study, fifteen New Zealand white rabbits were divided into five equal groups. An I-shaped or L-shaped thermoseed was inserted in each rabbit's liver, and then placed within the center of the magnetic induction coil during a 30-minute period of hyperthermia. The rabbits in the first group were sacrificed immediately after hyperthermia was induced once, while those in the other groups were sacrificed at 1, 3, and 7 days, respectively, also after one induction. The remaining three rabbits were sacrificed 4 days after three consecutive daily treatment sessions. The resected segments of liver were subsequently evaluated histopathologically for the extent of coagulation necrosis caused by heating of the thermoseed.
Results
The in-vitro study demonstrated that the temperature in the thermoseed, which was 25.9℃ before heating and 54.8℃ after heating, rose rapidly at first but progressively less rapidly as time elapsed. Light microscopic examination of the rabbits' livers revealed coagulation necrosis and infiltration by inflammatory cells around the insertion site of the thermoseed. The maximum diameter of coagulation necrosis was 2.81 ± 1.68 mm, and this occurred in the rabbits that were sacrificed 7 days after heat induction.
Conclusion
Needle-shaped duplex stainless steel thermoseeds show temperature-dependent-type heating characteristics, and in rabbit liver, induced coagulation necrosis of surrounding tissues after heat is applied for 30 minutes. These thermoseeds may thus be useful for the induction of interstitial hyperthermia.
The use of interstitial hyperthermia as a way of inducing localized hyperthermia in a limited tissue volume has been the subject of various studies investigating the treatment of cancer. Various induction methods have been developed (1, 2), and among these the radiofrequency electrode implantation technique has proven most popular and successful; the need to connect each implanted device to a power source limits its practicality, however, especially in the treatment of a deep-seated tumor. Heating a thermoseed by means of an external magnetic induction field has, though, been used to create localized interstitial hyperthermia without the need for a connection between the thermoseed and a power source.
Thermoseeds may be made from a variety of alloys, and their heat production properties thus vary accordingly, influencing the choice as to which is appropriate in each of a variety of specific applications. On the basis of their heatproduction characteristics, thermoseeds may be classified as one of three types (3): idealized constant-temperature thermoseeds, which maintain a constant Curie point in any environment; constant-power thermoseeds, which are assumed to produce constant power at any temperature and are made from stainless steel #433 or any ferromagnetic material with a Curie point well above the therapeutic temperature range; and temperature-dependent thermoseeds, which produce varying amounts of heat, depending on the environmental temperature. Most currently available thermoseeds, such as Ni-Cu, Ni-Pd, Pb-Co, and Ni-Si alloys, are of this type. Duplex stainless steel has a relatively high chromium content and relatively low nickel content, and in the presence of an external magnetic field produces heat in a temperature-dependent manner.
Our study reports the heat production characteristics of newly developed thermoseeds made from duplex stainless steel, and presents the results of in-vivo experiments to determine their thermal effects on normal rabbit liver.
Thermoseeds in the form of a 0.23-mm-diameter wire were made from Fe 55.1%, Cr 16.5%, Ni 9.5%, Nb 5.3%, Cu 4.0%, and Co 3.2%. They were either L-shaped and 4 cm long, with a right-angle bend 2.5 cm from one end for one-time delivery of interstitial hyperthermia, or I-shaped, 2.5 cm long, and suitable for complete embedding into the hepatic parenchyma of a rabbit (Fig. 1).
The apparatus required for magnetic field induction was an induction coil, a cooling system, and a magnetic field generator. The cylinder-shaped induction coil was 20 cm in length and 40 cm in diameter, and had nine turns of copper tube. Its maximum power was 7.0 kW, and its frequency, 114 kHz. For heating the thermoseeds, 5.4kW of energy (180 V and 30 A) was used.
To evaluate the heating characteristics of the thermoseeds, thermocouples were used to measure their temperature. A thermocouple is a temperature sensor made of two dissimilar metals, and is based on the principle that the junction between two metals generates a voltage that is a function of temperature. We prepared a plastic tube with three thermocouples passing through its wall. These, located at 0.5, 1.5, and 2.5 cm above the bottom of the tube, were connected to a personal computer for recording temperatures every second. Prior to the insertion of a 2.5-cm-long thermoseed, the tube was filled with 0.7 mL of water, and to minimize heat exchange with the surroundings, was embedded in a block of polystyrene foam placed in the center of the induction coil parallel to the long axis of its magnetic field (Fig. 2). The magnetic field was produced by 77% power output of the AC generator, the external magnetic field being supplied with power at 5.4kW for 30 minutes.
Fifteen New Zealand white rabbits were divided into five equal groups. Immediately, or at 1, 3, and 7 days after hyperthemia was induced once in groups I, II, III, and IV, respectively, the animals were sacrificed; in group V, this occurred 4 days after the last of three consecutive daily treatment sessions.
Each rabbit was anesthetized with a combination of Ketamine (15 mg/Kg; Ketara®, Yuhan, Seoul, Korea) and Xylazine (5 mg/Kg; Rompun®, Bayer Korea, Kyunggi-do, Korea) via intramuscular injection, and after three to five minutes, was placed in the supine position on an operating table. Inhalation anesthesia with isoflurane (Aerane®, Ilsung, Seoul, Korea) was administered during the procedure. After the rabbit was adequately anesthetized, the liver was exposed through midline incision. Its left lobe was selected and a thermoseed was inserted caudocranially through its inferior edge. The use of a needle holder for manipulation of the thermoseed facilitated accurate and convenient insertion, and for easy removal after hyperthermia, L-shaped thermoseeds were used in groups I-IV. A long-limb thermoseed (2.5 cm in length) was inserted into the liver, whereas a short-limb thermoseed (1.5 cm) was placed on the hepatic surface, without fixation (Fig. 3). I-shaped thermoseeds were used in group V, and were completely embedded within the hepatic parenchyma. After insertion of a thermoseed, the peritoneum was closed and wet gauze was placed on the skin to prevent dehydration. To create interstitial hyperthermia, the rabbit was placed in the central area of the induction coil (Fig. 4); the power and duration of the induced magnetic field were the same as in the in-vitro experiment. Before each rabbit was sacrificed, blood samples were obtained; death was induced by intravenous injection of 10 ml KCl within seven days of the implantation and heating procedures. The animals were autopsied immediately, and the resected hepatic segments were placed in a 10% buffered formalin solution. Representative specimens were sectioned perpendicular to the pathway of the thermoseed, and stained with hematoxyin-eosin. An anatomic pathologist evaluated all specimens to interpret the histopathologic changes caused by hyperthermia. Examination in a low-power microscopic field demonstrated the extent of coagulation necrosis, and this was measured at the longest diameter using an electronic scale. For each animal, complete blood count and liver function tests were performed, and the results were compared with those of normal rabbit blood. Student's t test was used for statistical analysis.
Over the 30-minute period that the external magnetic induction field was applied, the temperature rose from 25.9℃ before heating to 54.8℃ after. Mean temperatures measured by three thermocouples were 29.5℃ before heating, 36.5℃ at 5 minutes, 43.3℃ at 10 minutes, 47.9℃ at 15 minutes, 51.2℃ at 20 minutes, 53.4℃ at 25 minutes, and 54.8℃ at 30 minutes (Table 1). The temperature rose rapidly at first, but more slowly as time elapsed. During heating, the thermocouple located in the lowest portion of the plastic tube showed the highest temperature, and the one in the highest portion, the lowest. The mean temperature difference between the lowest and highest thermocouples was 4.3℃, but such differences also tended to decrease with time.
We found histopathologic evidence of thermal change in 14 of 15 rabbits, with no abnormal changes in one rabbit in group I (the immediate post-hyperthermia group), probably due to an error in resection of the specimen. Light microscopic examination after fixation in formalin solution for 24 hours showed coagulation necrosis and infiltration by inflammatory cells around the thermoseed insertion site. Histopathologic examination showed that an insertion site was either an empty space, or was filled with hemorrhage or necrotic tissue.
Coagulation necrosis occurred around the insertion site to a variable extent, but was most severe at the central portion (Fig. 5). In pathologic specimens, such areas were either irregular and lobulated, or stellate shaped, rather than oval or round. The mean extent of coagulation necrosis, as measured by light microscopic examination, was 0.88 ± 0.13 mm in group I (n=2), 1.53 ± 0.14 mm in group II (n=3), 2.21 ± 0.23 mm in group III (n=3), 2.81 ± 1.68 mm in group IV (n=3), and 1.75 ± 0.66 mm in group V (n=3) (Table 2). In the groups in which hyperthermia was induced once, the extent of coagulation necrosis tended to increase with time after heat was applied. Fibrosis was noted in groups III and IV, but was more severe in group IV. The extent of infiltration by inflammatory cells was most severe in group III, followed by group IV and group II.
The blood count of pre- and post-hyperthermia rabbits revealed no statistically significant differences, though the liver function test showed that in group II, alanine aminotransferase (ALT) was significantly elevated. Mean ALT values in pre- and post-hyperthermia rabbits were 40 U/L and 95 U/L, respectively.
Ferromagnetic alloys become heated by eddy current loss when placed in an oscillating magnetic induction field. Heat is transferred to the surrounding tissue, resulting in localized interstitial hyperthermia. The temperature at which the alloy changes from the ferromagnetic to the nonmagnetic or paramagnetic state is termed its Curie point. A needle-shaped alloy used in the induction of localized interstitial hyperthermia is known as a thermoseed, and magnetic induction heating of thermoseed implants can be used to produce highly localized hyperthermia in deep-seated tumors. Thermoseeds absorb energy from an externally applied magnetic induction field without being in direct contact with the source of power. Most thermoseeds lose their ferromagnetic properties at a predetermined temperature (Curie point) within the therapeutic temperature range, and automatic temperature regulation can thereby be achieved.
Hyperthermic treatment using thermoseed implants requires their correct insertion into the target tissue, which can be difficult, but these implants have several advantages: the procedure does not require any wire connection between the implants and external devices; it produces automatic temperature control in the target tissue if self-regulating thermoseeds are used; and target tissue can be heated repeatedly without the need for any additional devices or surgery. Metal thermoseeds generate heat by eddy current loss, and tissues near the thermoseeds are then heated via thermal conduction.
In the hyperthermic treatment of tumors by thermoseeds, thermal self-regulation should be particularly beneficial. Self-regulating thermoseeds adjust their rate of heat production so as to maintain a temperature close to the Curie point. Thus, if an array of thermoseeds were used for interstitial hyperthermia treatment of a tumor, the temperature attained by each implant would depend on its particular environment (4).
Several materials that generate heat in the presence of a magnetic field have been developed. Various combinations of ferromagnetic alloys such as Ni-Cu, Ni-Pd, Ni-Co, and Ni-Si have been used as self-regulating thermoseeds, the size and shape of which have been appropriate for special application to deep-seated tumors (5-8). Because thermoseeds with a Curie point within the therapeutic range contain nickel, copper, and sometimes other potentially toxic elements, biocompatibility has been a concern in the development of thermoseeds for therapeutic use. Efforts to render thermoseeds nontoxic have involved several approaches, including coating the seed with layers of biocompatible material, or encasing it within a catheter. However, the coating materials are not permanent and can influence the heating characteristics or change the conduction of heat within tissue. Another problem is that thermoseeds made of Ni-Co are too soft to implant into tissue, so steel needles that act as a temporary conduit are required (9).
Because of the advantages offered by self-regulation at a defined Curie point, little attention has been devoted to the use of relatively biocompatible implants made of stainless steel. This is relatively safe in the body, so has been used widely in medical applications; because its Curie point is beyond the therapeutic range, it is not, though, a suitable thermoseed material, and depending on its composition, may also be incapable of producing sufficient heat. Duplex stainless steel, however, has both magnetic and nonmagnetic heating characteristics, and if these are combined appropriately, is suitable for use within the therapeutic temperature range and in the presence of an induced magnetic field.
We evaluated the heating characteristics of thermoseeds made of duplex stainless steel in both in-vivo and in-vitro experiments. In the latter, temperatures were measured during the heating of duplex stainless steel thermoseeds by induced magnetic field, and the resultant time-temperature curve showed heating characteristics that were temperature dependent rather than of the constant power type. The histopathologic study of rabbit liver after hyperthermia showed coagulation necrosis, infiltration by inflammatory cells, and fibrosis around the insertion site of a thermoseed.
The responses of tissue or cells to hyperthermic treatment depend on the degree and duration of heating. Cellular homeostasis can be maintained by slight temperature increases (to approximately 40℃), but beyond this, at 42-45℃, cells become more susceptible to damage by other agents such as chemotherapy and radiation (10). When temperatures are increased to 46℃ for 60 minutes, irreversible damage occurs (11), and temperatures of 50-52℃ markedly shorten the time required to induce cytotoxicity (4-6 minutes) (12). A key aim of ablative therapies is to achieve and maintain a 50-100℃ temperature range throughout the entire target volume (1): in our experiments, a temperature of over 50℃ was maintained for 12 minutes during heating which lasted 30 minutes.
Duplex stainless steel thermoseeds of a relatively small diameter provide adequate increases in temperature (13), and compared with those which are of large diameter, their use confers certain advantages: heat production by thermoseeds is improved by the application of a number of filaments with a small diameter rather than a single solid cylindrical thermoseed with the same sectional area (14), and less tissue damage is also expected. Even though our in-vivo study showed a relatively small extent of coagulation necrosis, this problem can, in theory, be overcome by increasing the radius of the thermoseed incrementally, using multiple thermoseeds simultaneously in a square grid pattern, prolonging heating time, using a stronger magnetic induction field, or using thermoseeds with a higher Curie point.
The temperature reached by a thermoseed is a function of several variables, including the strength and frequency of the electromagnetic field, the orientation of the thermoseed within the coil, its proximity to other thermoseeds, the permeability and electrical conductivity of the thermoseed, the local blood perfusion rate, and the thermoseed's thermophysical properties (15, 16). By suitably adjusting some of these variables, it is possible to achieve the temperature which induces the required degree of hyperthermia in target tissue. If small-diameter thermoseed wires made of duplex stainless steel are used as stent material, it is likely that inhibition of intimal growth (in the case of a vascular stent), or reduction in the size of a malignant tumor (in the case of a biliary stent), can be achieved by heating the thermoseed with an externally induced magnetic field, without the need for additional surgery.
Our study suffers several limitations. First, no direct comparison with other self-regulating thermoseeds was made; second, in terms of the diameter of the thermoseeds, the power of the magnetic induction field, and the duration of heating, our in-vitro and in-vivo experiments were conducted under only one set of conditions; third, the duration of the in-vivo experiments was too short to evaluate the biocompatiblity of the thermoseeds; fourth, only hepatic tissue was used for the in-vivo experiments.
In conclusion, newly developed duplex stainless steel thermoseeds demonstrate adequate temperature and temperature-dependent heating characteristics in in-vitro experiments, and induce coagulation necrosis in rabbit liver in in-vivo experiments. Duplex stainless steel is thus a suitable new thermoseed material for the induction of interstitial hyperthermia.
Figures and Tables
 | Fig. 1Two thermoseeds made from duplex stainless steel: L-shaped and I-shaped, both are 2.5 cm in length. |
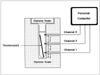 | Fig. 2Method of temperature measurement for in-vitro experiment. An I-shaped thermoseed is located within a test tube filled with 0.7 mL of water. Three thermocouples, connected to a personal computer for data acquisition, are attached to the inside wall of the plastic tube. |
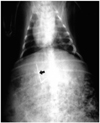 | Fig. 3Radiograph of a rabbit shows an L-shaped thermoseed (arrow) in the liver. The inserted portion of the thermoseed is aligned parallel to the long axis of the rabbit. |
 | Fig. 4Heating system for the induction of interstitial hyperthermia in rabbit liver, using a thermoseed.
A. Schematic drawing shows the heating system and a rabbit with an L-shaped thermoseed for the induction of interstitial hyperthermia.
B. Photograph of rabbit and induction coil during interstitial hyperthermia. A mask and equipment for general anesthesia of the animal are visible.
|
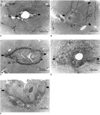 | Fig. 5Light micrographs of rabbit liver after the induction of interstitial hyperthermia using a duplex stainless steel thermoseed. The area of coagulation necrosis is indicated by arrows.
A. Immediately after heating, chromatin condensation of the nucleus and increased eosinophilia of hepatocytic cytoplasm around the central hole (thermoseed insertion site) are noted (original magnification, ×40; hematoxylin-eosin staining).
B. A day after heating, a thin band of coagulation necrosis around the central hole (filled with RBCs and necrotic tissue) and infiltration of some eosinophils at the margin of coagulation necrosis are apparent (original magnification, ×40; hematoxylin-eosin staining).
C. Three days after heating, an area of coagulation necrosis larger than that seen in Fig. 5B, together with inflammatory cell infiltration and early fibrosis around the area of necrosis, is seen (original magnification, ×40; hematoxylin-eosin staining).
D. Seven days after heating, an extensive area of coagulation necrosis, about 4 mm in diameter and with extensive surrounding fibrosis, is noted (original magnification, ×20; hematoxylin-eosin staining).
E. Four days after three consecutive daily periods of heating, calcified hyaline material in the area of coagulation necrosis is seen, together with multinucleated giant cell infiltration. There is extensive fibrosis around the necrotic area, but surrounding inflammatory cells are not similarly extensive (original magnification, ×40; hematoxylin-eosin staining).
|
References
1. Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR. 2000. 174:323–331.
2. Lim HK. Radiofrequency thermal ablation of hepatocellular carcinomas. Korean J Radiol. 2000. 1:175–184.
3. Brezovich IA, Ruby FM. Practical aspect of ferromagnetic thermoseed hyperthermia. Radiol Clin North Am. 1989. 27:589–602.
4. Stauffer PR, Cetas TC, Jones RC. Magnetic induction heating of ferromagnetic implant for inducing localized hyperthermia in deep-seated tumors. IEEE Trans Biomed Eng. 1984. 31:76–90.
5. Brezovich IA, Atkinson WJ. Temperature distribution in tumor model heated by self-regulating nickel-copper alloy thermoseed. Med Phys. 1984. 11:145–152.
6. Matsumoto M, Yoshimura N, Honda Y, Hiraoka M, Ohura K. Ferromagnetic hyperthermia in rabbit eyes using a new glass-ceramic thermoseed. Graefes Arch Clin Exp Ophthalmol. 1994. 232:176–181.
7. Molloy JA, Ritter RC, Grady MS, Howard MA, Quate EG, Gillies GT. Experimental determination of the force required for insertion of a thermoseed into deep brain tissue. Ann Biomed Eng. 1990. 18:299–313.
8. Takegami K, Sano T, Wakabayashi H, et al. New ferromagnetic bone cement for local hyperthermia. J Biomed Mater Res. 1998. 43:210–214.
9. Meredith RF, Brezovich IA, Weppelmann B, et al. Ferromagnetic thermoseed: suitable for an afterloading interstitial implant. Int J Oncol Biol Phys. 1989. 17:1341–1346.
10. Seegenschmiedt MH, Brady LW, Sauer R. Interstitial thermoradiotherapy: review of technical and clinical aspect. Am J Clin Oncol. 1990. 13:352–363.
11. Larson TR, Bostwick DG, Corica A. Temperature-correlated histopathologic changes following microwave thermoablation of obstructive tissue in patients with benign prostatic hyperplasia. Urology. 1996. 47:463–469.
12. Goldberg SN, Hahn PF, Helpern EF, Fogle RM, Gazelle GS. Radio-frequency tissue ablation: effect of pharmacologic modulation of blood flow on coagulation diameter. Radiology. 1998. 209:761–767.
13. Wieringen N, Dijk JDP, Snel CE, Cetas TC. Power absorption and temperature control of multi-filament pallidium-nickel thermoseed for interstitial hyperthermia. Phys Med Biol. 1996. 41:2367–2380.
14. Wieringen N, Kotte ANT, Leeuwen GMJ, Lagendijk JJW, Dijk JDP, Nieuwenhuys GJ. Dose uniformity of ferromagnetic seed implant in tissue with discrete vasculature: a numerical study of the impact of seed characteristics and implantation techniques. Phys Med Biol. 1998. 43:121–138.
15. Tompkins DT, Vanderby R, Klein SA, Beckman WA, Steeves RA, Pailwal BR. Effect of interseed spacing, tissue perfusion, thermoseed temperatures and catheters in ferromagnetic hyperthermia: results from simulations using finite element models of thermoseed and catheters. IEEE Trans Biomed Eng. 1994. 41:975–985.
16. Atkinson W, Brezovich IA, Chakraborty DP. Usable frequencies in hyperthermia with thermal seeds. IEEE Trans Biomed Eng. 1984. 31:70–75.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download