Abstract
Objective
To evaluate the efficacy of newly designed covered and non-covered coated colorectal stents for colonic decompression.
Materials and Methods
Twenty-six patients, (15 palliative cases and 11 preoperative) underwent treatment for the relief of colorectal obstruction using metallic stents positioned under fluoroscopic guidance. In 24 of the 26, primary colorectal carcinoma was diagnosed, and in the remaining two, recurrent colorectal carcinoma. Twenty-one patients were randomly selected to receive either a type A or type B stent; for the remaining five, type C was used. Type A, an uncovered nitinol wire stent, was lightly coated to ensure structural integrity. Type B (flare type) and C (shoulder type) stents were polyurethane covered and their diameter was 24 and 26mm, respectively. The rates of technical success, clinical success, and complications were analyzed using the chi-square test, and to analyse the mean period of patency, the Kaplan-Meier method was used.
Results
Thirty of 31 attempted placements in 26 patients were successful, with a technical success rate of 96.8% (30/31) and a clinical success rate of 80.0% (24/30). After clinically successful stent placement, bowel decompression occurred within 1-4 (mean, 1.58 ± 0.9) days. Five of six clinical failures involved stent migration and one stent did not expand after successful placement. In the preoperative group, 11 stents, one of which migrated, were placed in ten patients, in all of whom bowel preparation was successful. In the palliative group, 19 stents were placed in 15 patients. The mean period of patency was 96.25 ± 105.12 days: 146.25 ± 112.93 for type-A, 78.82 ± 112.26 for type-B, and 94.25 ± 84.21 for type-C. Complications associated with this procedure were migration (n=6, 20%), pain (n=4, 13.3%), minor bleeding (n=5, 16.7%), incomplete expansion (n=1, 3.3%), and tumor ingrowth (n=1, 3.3%). The migration rate was significantly higher in the type-B group than in other groups (p=0.038).
Conclusion
Newly designed covered and non-covered metallic stents of a larger diameter are effective for the treatment of colorectal obstruction. The migration rate of covered stents with flaring is higher than that of other types. For evaluation of the ideal stent configuration for the relief of colorectal obstruction, a clinical study involving a larger patient group is warranted.
In advanced colorectal cancer, obstruction is a common complication, particularly if the lesion is located in the left colon (1-5). In recent years, some authors have reported favorable results of the nonsurgical treatment of colonic obstruction using an uncovered expandable metallic stent 10-22 mm in diameter (1-12). The placement of an uncovered self-expanding metallic stent, a minimally invasive procedure, can be performed without general anesthesia and reduces the need for colostomy (8). It provides temporary decompression of the colon before single-stage surgery, or may be used for palliation. Patients undergoing palliative treatment have, however, developed new colonic obstructions or tumor growth into the stent (7), and additional complications have included stent migration, pain, bleeding, and bowel perforation (1-12). To prevent tumor ingrowth and to treat fistulae, Choo et al. applied a polyurethane covering to the metallic stent but migration and problems with the introductory system persisted (7). The purpose of this prospective study was to implement the use of newly developed larger diameter self-expandable covered and non-covered stents, and to evaluate their efficacy in the treatment of colorectal obstructions.
Between May 1999 and July 2000, 26 consecutive patients were treated for the relief of colorectal obstruction via fluoroscopically guided anal intubation of expandable metallic stents of three different types. Pre-procedural informed consent was obtained from all patients. Stent placement was for palliative care in 15 cases and preoperative treatment in 11. In 24 patients the diagnosis was primary colorectal carcinoma, and in the other two, recurrent colorectal carcinoma. A diagnosis of colonic obstruction was initially established by means of the patient's clinical history, rectal examination, and conventional abdominal radiography. To determine whether an obstructive mass was present, and if so, its precise location, computed tomography (CT) followed by barium-enema or water-soluble contrast enema examination, or both, was then performed. Twenty-one patients were randomly selected to receive either a type-A or type-B stent, and for the remaining five, type C was used.
At the beginning of the study only two types of colorectal stent, made of lightly coated wire (type A)or fully polyurethane covered (type B), were designed and constructed. In order to decrease migration, type C was devised later (Fig. 1). Stent length averaged 8.4 (range, 6-14) cm.
The type-A stent, 30 mm in diameter and with its ends flared to 40mm to prevent migration, was woven from a single thread of 0.25-mm nitinol wire in a tubular configuration. It was lightly coated with a 12% polyurethane solution (Biospan; Polymer Technology Group, Emeryville, Cal., U.S.A.), and a dipping method was used to increase its expansile power by fixing the crossing of the wire. It was loaded in a 4-mm introducer for deployment.
The type-B stent consisted of several 3-cm-long segments, each woven from a single thread of 0.25-mm nitinol wire in a tubular configuration. The body of each segment was 24 mm in diameter, with the ends flared to 34 mm. To cover the segments, an Epolene wax mold (Eastman Chemical, Kingsport, Tenn., U.S.A.) was used. Several segments were set on its surface, and it was then dipped into the polyurethane solution and dried in an oven for 24 hours. One hundred percent nylon mesh was used to secure the segment connections.
The type-C stent, also polyurethane covered, was made from several 2-cm-long segments knitted from a single thread of 0.25-mm nitinol wire in a tubular configuration and an interlocking diamond-shaped pattern. This stent consisted of three parts: a head and a tail (both 40 mm in diameter) and a body (26 mm in diameter). The head and tail were connected at right angles to the body, and covered with a 12% polyurethane solution using the same technique employed for type B. Stent types B and C were loaded in a 7-mm introducer.
So that types B and C could be removed, a 2-mm-diameter nylon monofilament loop was placed inside each bend and secured with sutures (Fig. 1). These loops formed the anchor for another nylon thread which was passed through each of them to form a large loop or drawstring that filled the inner circumference of the inside of the distal stent. The resultant loop was then tied at the upper inner margin of the stent.
Before use, stent hoop strength was measured to compare radial force. HD25 was defined as the measured hoop strength when a stent's circumference was decreased to 75% of its baseline length, and as HD50 when the circumference was decreased to 50%. A 10-mm-wide strip was used to apply stress to each stent: for types A, B and C, HD25 was 270, 460, and 150 grams force, respectively, while for HD50, the respective readings were 530, 690, and 280 (Table 1).
Tumor location was determined using a barium-sulfate suspension or water-soluble contrast material. Under fluoroscopic guidance, a 145-cm-long, 0.038-inch-diameter hydrophilic guidewire (Terumo, Tokyo, Japan) was advanced across the obstruction, allowing an angiographic catheter to be positioned above it. The guidewire was then removed, and the length and position of the obstruction were determined by the injection of contrast material prior to stent placement. A 0.038-inch-diameter stiff guide wire (Amplatz Superstiff; Meditech/Boston Scientific, Watertown, Mass., U.S.A.) was then introduced. Under fluoroscopic guidance, the whole introductory assembly (a sheath, a compressed stent, and a pusher catheter) was passed over the guidewire into the rectum and advanced until the distal tip of the stent reached approximately 2 cm beyond its stricture. The pusher catheter was held in place with one hand while the sheath was slowly withdrawn in a continuous motion with the other. This freed the stent, allowing it to lie within the stricture and expand. Technical success was defined as successful placement of the stent at the lesion site.
To determine the stent's position and the relief of colonic obstruction until the patient was discharged, a conventional radiograph of the abdomen was obtained and changes in bowel gas patterns were analyzed within 24 hours of stent placement and each subsequent day thereafter. Clinical failure was defined as the failure of decompression (including stent migration) after successful stent placement. If surgery was indicated, the patient underwent elective surgical resection of the tumor, with primary anastomosis of the colon. Patients in the palliative group underwent clinical follow-up, conventional abdominal radiographs being obtained at monthly intervals. In one patient in whom a type-A stent was placed, endoscopy was performed to evaluate the cause of symptom recurrence. Period of patency was defined as the period from stent placement to the recurrence of symptoms of obstruction in clinically successful cases. The rate of technical success, clinical success and complications were analyzed using the chi-square test, and to analyze the mean period of patency, the Kaplan-Meier method was used. The confidence interval was 95%.
Thirty (8 type-A; 16 type-B; and 6 type-C stents) of 31 attempted placements in 26 patients were successful, with a technical success rate of 96.8% (Table 2). Despite the integration of fluoroscopic and endoscopic guidance, we were unable to advance a guidewire through the obstructed distal ascending colon in one patient (#18). The clinical success rate was 80.0% (24/30): 100% for type A, 68.8% for type B, and 83.3% for type C (Table 3); the differences were statistically insignificant (p=0.191). In 24 clinically successful stent placements, bowel obstructions were resolved within 1-4 (mean, 1.58 ± 0.9) days of placement. In six cases of clinical failure, five stents had migrated and one did not expand after successful placement. In the 24 clinically successful instances, patients were able to defecate frequently, though seven complained of abdominal discomfort.
In the preoperative group, 11 (4 type-A, 5 type-B and 2 type-C) stents were placed in ten patients; one received two stents. In all patients in whom stents were placed, bowel preparation was successful and at the placement site, the colon was clean. Surgery was performed 2-9 (mean, 5 ± 2.07) days after stent insertion, and no complications, such as wound infection or abdominal abscess formation, occurred (Fig. 2).
In the palliative group, 19 stents (4 type-A, 11 type-B, 4 type-C) were placed in 15 patients. The mean period of patency was 96.25 ± 105.12 days: 146.25 ± 112.93 for type-A, 78.82 ± 112.26 for type-B, and 94.25 ± 84.21 for type-C; the differences were statistically insignificant (p=0.576) (Table 4). Six placements in five patients were clinically unsuccessful: five involved stent migration and in one, the stent failed to expand completely. In two patients (#1, 3), two type-B stents were placed, but all four migrated within 1.3 ± 0.5 (range, 1-2) days of placement. In one patient (#1) bowel obstruction was relieved after the second migration, and the symptoms associated with obstruction did not develop for seven months, at which point a T-loop colostomy was performed. In patient #11, a type-A stent was placed after a type-B migrated, and this led to the relief of obstruction. In the remaining (#24), the type-C stent used did not fully expand and a T-loop colostomy was performed. At 180 and 79 days, respectively, after insertion, two of the clinically improved patients (#8, 25) underwent T-loop colostomy or ileostomy because of symptom recurrence due to tumor overgrowth (Fig. 3).
The overall complication rate was 50.0%: 50% for type A, 56.3% for type B, and 33.3% for type C. The complications involved were migration (n=6, 20.0%), pain (n=4, 13.3%), minor bleeding (n=5, 16.7%), incomplete stent expansion (n=1, 3.3%), and tumor ingrowth (n=1, 3.3%) (Table 4). The migration rate was significantly higher in the type-B group (p=0.038), while minor bleeding developed frequently in the type-C group (p=0.037). Two of nine patients with primary rectal cancer and two of 12 with rectosigmoid colon cancer complained of anal pain after stent placement. Two in whom a type-A stent had been placed complained of pain, which in one case was severe; those who underwent type-B stent placement complained of pain in one case, and minor bleeding in two. Type-C stents caused pain in one case and minor bleeding in three, and in one other there was incomplete expansion. Patient #1 underwent emergency colostomy seven months after stent migration. The type-B stent placed in patient #11 migrated nine days after placement, and was replaced with a type-A stent; relief from bowel obstruction lasted 27 days. In patients #21, in whom a type-A stent was inserted, obstruction recurred due to tumor ingrowth and was confirmed by endoscopy. We placed an additional type-C stent inside the type-A stent, and until her death, this patient experienced no further bowel obstruction. (Fig. 4). In patient #23, who underwent type-C stent insertion, another stricture was discovered just below the stent. We removed this, using a hook, replacing it with a type C, which was longer.
Malignant colonic tumors, which cause acute obstruction, are often difficult to treat. They have traditionally required emergency surgical decompression by colostomy, followed by resection, a procedure that carries a high rate of morbidity and mortality (7-9). Benign strictures occurring after radiation therapy, surgery (such as colostomy repair), or end-to-end anastomosis of the large intestine increase the difficulty of treatment, and the avoidance of a permanent colostomy, by means of rectal conservation, is also an important quality-of-life issue (5). For these reasons, the use of minimally invasive treatment techniques that reduce the need for, or supplement, surgery and emphasise quality of life needs to be explored.
The placement of an expandable metallic stent is an alternative option for the treatment of acute colonic obstruction (7-12). The self-expanding stents currently available have great elasticity, which allows them to flex along the longitudinal axis and adapt to the angles of the intestinal lumen. Moreover, they maintain their most recent form after being inserted across a stenosis, exercising only radial pressure (10). Although a statistically significant benefit arising from the use of the large 22-mm-diameter Wallstent placed by Mainar et al. (5) has not, to our knowledge, been proven, the use of a large-diameter self-expanding stent may decrease the risk of stent dysfunction due to stool impaction or mucosal prolapse, particularly in cases involving palliative placement. A new self-expanding metal stent with an internal diameter of 24 to 30 mm might broaden the clinical indications for this therapy (11). In our study, covered stents with an internal diameter of 24-26 mm, and non-covered, coated stents with an internal diameter of 30 mm were used. In the palliative group, symptoms of obstruction recurred in two of 14 clinically successful placements, though in the remaining 12 such placements, patients were free of such symptoms until their death. For the palliation of colonic obstruction, metallic stents of a larger diameter were effective. Among the three types of stent, differences in the mean period of patency were not statistically significant (p =0.576).
The complications reported after stent placement have included stent migration, pain, bleeding, bowel perforation, tumor ingrowth, and new obstructions (7). In our study, tumor ingrowth was confirmed by fluoroscopy in one patient. We inserted an additional covered stent inside the one which was non-covered, and the patient was subsequently able to defecate well until her death. Minor bleeding developed in five patients in the covered stent group: because a larger introductory system is required, the placement of such stents may be more traumatic. Severe pain developed in one patient in the non-covered stent group, and could not controlled by medication. We believe that in this case the stent should have been removed, but once a non-covered stent is in position, this is not easy. Because of continuous stress on a tumor, that can give rise to stretching pain, the use of stents of a larger diameter did tend to cause pain. In our study, the incidence of this was highest when type-A stents were used, though among different stent types the differences in this regard were not statistically significant.
The HD50 of type-A, -B, and -C stents was 530, 690, and 280 grams of force, respectively. In patient 24, who underwent type-C stent insertion, conventional follow-up radiography revealed incomplete stent expansion, and during the eight-day follow-up period, at the end of which a T-loop colostomy was performed, the patient produced only a small amount of feces. In this case, the type-C stent should have been removed, prior to the insertion of one which was more powerful, such as the type B. In 24 instances of clinically successful stent placement, conventional radiography or fluoroscopic examination demonstrated that in each case, stent expansion was almost complete. All three types of colorectal stents showed good longitudinal flexibility and incorporated anti-migration features such as flaring or shouldering. In our study, the type-C stent had a lower migration rate than the type-B, and according to our results, shouldering might be more effective than flaring. In addition, a stent body constructed in such a way that gaps between segments are small effectively minimizes migration and improves longitudinal flexibility.
The selection of stent type depended on tumor location and size. A non-covered stent could be introduced as far as the distal ascending colon, but because of its large and rigid introductory catheter, placement of a covered stent proximal to the descending colon was difficult. A stent which is covered can be safely positioned, and one which is retrievable can easily be repositioned or removed if a complication arises. In addition, spillage of the contents of a ruptured large bowel can be prevented (13-15).
This study suffers several limitations. First, patient selection was incompletely randomized and there might thus have been a selection bias. Second, our study design was less than fully appropriate. The migration rate of type-B stents was higher than expected, but should have been preevaluated by means of a pilot study. Finally, the number of patients involved was too small to fully evaluate the statistical significance of the findings.
In conclusion, newly designed self-expanding metallic stents of a larger diameter (24-30 mm) were effective for the palliation or preoperative decompression of obstructive symptoms. Where covered stents with flaring were used, the migration rate was higher. Differences in the mean patency period between the three types of stent were not statistically significant. For evaluation of the ideal stent configuration for the treatment of colorectal obstruction, a clinical study involving a larger group of patients is warranted.
Figures and Tables
Fig. 1
A. Three types of colorectal stent: type A (upper), type B (middle), and type C (lower).
B. The drawstring. Each bend of each segment contains a 2-mm-diameter nylon monofilament loop secured with sutures (thin arrow). These loops form the anchor for another nylon thread which is passed through each of them to form a large loop or drawstring (thick arrow) that fills the inner circumference of the inside of the proximal stent. The resultant loop is then tied at the stent's upper inner margin.

Fig. 2
Type-A stent placement for presurgical bowel preparation (patient #10).
A. Irregular narrowing of the proximal rectum (arrow) is apparent.
B. Although focal narrowing remains, good passage of contrast medium is observed after type-A stent placement. Four days after placement, this patient underwent anastomosis and tumor resection.
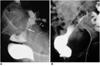
Fig. 3
Type-B stent placement for palliation (patient #8).
A. Irregular narrowing of the rectosigmoid colon (arrow) can be seen.
B. After type-B stent placement, good patency is observed (arrow). In this patient, symptoms of obstruction recurred 180 days after placement, and a palliative ileostomy was performed.
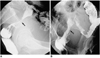
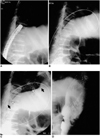
Fig. 4
Type-C stent placement inside a type-A stent (patient #21).
A. Tumor ingrowth, which developed four months after stent placement, was confirmed by endoscopy.
B. Guidewire advanced through previously placed type-A stent.
C. Deployment of type-C stent (arrow), which is longer than type A (arrowheads).
D. The type-C stent showed good patency and was patent for three months, until the patient's death.
References
1. Camunez F, Echenagusia A, Simo G, Turegano F, Vazquez J, Barreiro-Meiro I. Malignant colorectal obstruction treated by means of self-expanding metallic stents: Effectiveness before surgery and in palliation. Radiology. 2000. 216:492–497.
2. Mainar A, Ariza MADG, Tejero E, et al. Acute colorectal obstruction treatment with self-expandable metallic stents before scheduled surgery: results of a multicenter study. Radiology. 1999. 210:65–69.
3. De Gregorio MA, Mainar A, Tejero E, et al. Acute colorectal obstruction: stent placement for palliative treatment: results of a multicenter study. Radiology. 1998. 209:117–120.
4. Saida Y, Sumiyama Y, Narao J, Takase M. Stent endoprosthesis for obstructive colorectal cancers. Dis Colon Rectum. 1996. 39:552–555.
5. Loggie BW. Surgical concept in the treatment of colorectal cancer. Semin Roentgenol. 1996. 31:111–117.
6. Richards F, Richards CP. Colorectal cancer: etiologic and clinical aspects. Semin Roentgenol. 1996. 31:103–110.
7. Choo IW, Do YS, Suh SW, et al. Malignant colorectal obstruction: treatment with a flexible covered stent. Radiology. 1998. 206:415–421.
8. Binkert CA, Ledermann H, Jost R, Saurenmann P, Decurtins M, Zollikofer CL. Acute colonic obstruction: clinical aspects and cost-effectiveness of preoperative and palliative treatment with self-expanding metallic stents: a preliminary report. Radiology. 1998. 206:199–204.
9. Canon C, Baron TH, Morgan DE, Dean PA, Koehler RE. Treatment of colonic obstruction with expandable metal stents: radiologic features. AJR. 1997. 168:199–205.
10. Spinelli R, Fante MD, Mancini A. Self-expanding mesh stent for endoscopic palliation of rectal obstructing tumors: a preliminary report. Surg Endosc. 1992. 6:72–74.
11. Rey JF, Romanczyk T, Greff M. Metal stent palliation of rectal carcinoma: a preliminary report on 12 patients. Endoscopy. 1995. 27:501–504.
12. Mainar A, Tejero E, Maynar M, Ferral H, Castaneda-Zuniga W. Colorectal obstruction: Treatment with metallic stents. Radiology. 1996. 198:761–764.
13. Grunshaw ND, Ball CS. Palliative treatment of an enterorectal fistula with a covered metallic stent. Cardiovasc Intervent Radiol. 2001. 24:438–440.
14. Song HY, Lee DH, Seo TS, et al. Retrievable covered nitinol stents: experiences in 108 patients with malignant esophageal strictures. J Vasc Interv Radiol. 2002. 13:285–293.
15. Tsunoda S, Shimada Y, Watanabe G, Nakau M, Imamura M. Covered metallic stent treatment of a patient with spontaneous rupture of the esophagus. Dis Esophagus. 2001. 14:254–257.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download