Abstract
Objective
To compare the use of conventional, real-time compound, and pulse-inversion harmonic imaging in the evaluation of breast nodules.
Materials and Methods
Fifty-two breast nodules were included in this study, conducted between May and December 2000, in which conventional, real-time compound, and pulse-inversion harmonic images were obtained in the same plane. Three radiologists, each blinded to the interpretations of the other two, evaluated the findings, characterizing the lesions and ranking the three techniques from grade 1, the worst, to grade 3, the best. Lesion conspicuity was assessed, and lesions were also characterized in terms of their margin, clarity of internal echotexture, and clarity of posterior echo pattern. The three techniques were compared using Friedman's test, and interobserver agreement in image interpretation was assessed by means of the intraclass correlation coefficient.
Results
With regard to lesion conspicuity, margin, and internal echotexture of the nodules, real-time compound imaging was the best technique (p < 0.05); in terms of posterior echo pattern, the best was pulse-inversion harmonic imaging (p < 0.05). Real-time compound and pulse inversion harmonic imaging were better than conventional sonography in all evaluative aspects. Interobserver agreement was greater than moderate.
Conclusion
Real-time compound and pulse-inversion harmonic imaging procedures are superior to conventional sonography in terms of both lesion conspicuity and the further characterization of breast nodules. Real-time compound imaging is the best technique for evaluation of the margin and internal echotexture of nodules, while pulse-inversion harmonic imaging is very effective for the evaluation of the posterior echo patterns.
Breast ultrasonography (US) can improve the accuracy of clinically and mammographically detected abnormalities. It has been used to differentiate solid from cystic breast nodules and benign from malignant solid nodules, and a problem-solving tool in the radiologist's armamentarium (1-17). A study by Stavros et al. (17) employing high-frequency, 7.5-10.0 MHz, near-field imaging probes for distinguishing between benign and malignant nodules showed very high sensitivity and negative predictive values of 98.4% and 99.5%, respectively. However, the positive predictive value, 38%, was very low. Thus, there is a need for an ultrasonographic approach, which in possible cases of breast cancer has a higher predictive value.
Two newer US techniques, namely harmonic imaging and real-time compound imaging (RTCI) are now in use. Harmonic US is a sensitive technique available for imaging nonlinear echoes that arise from tissue (18), and a number of recent articles have demonstrated the usefulness of pulse-inversion harmonic imaging (PIHI) in liver and kidney compared to conventional imaging (CI) (19, 20). RTCI obtains multiple coplanar images and combines them into a single image in real time. The prospective study of breast nodules has not, however, been reported.
The purpose of this study, then, is to compare CI, RTCI, and PIHI in terms of which is most suitable for the evaluation of breast nodules.
This study, conducted between May and December 2000, involved 28 consecutive patients aged 25 to 62 (mean, 45) years with 52 breast nodules. Six of these nodules were aspirated, and biopsy was performed on nine: pathologic examination revealed that among the 15, four were due to invasive breast cancer, one to atypical ductal hyperplasia, and the remaining ten were benign. Follow-up US involving 28 nodules and performed at a mean interval of 9.2 months, revealed no interval change, and on the basis of their imaging features we considered these nodules benign. After initial US, the remaining nine nodules were not investigated further. The nodules ranged in size from 3 to 20 (mean, 7.1) mm.
An HDI 5000 SonoCT (Advanced Technology Laboratories, Bothell, Wash., U.S.A.) was used, together with a broad-bandwidth 12-5 MHz, linear scanhead. Breast nodules were scanned by CI first, then by RTCI and PIHI in the same plane and with the same parameters including magnification, depth, focus, and tissue compression. In each mode, gray-scale gain was adjusted for. Our US equipment can operate in two RTCI modes: target and survey. The former produces three coplanar images, and the latter, nine. In this study, the survey mode was used, and scanning was performed by one of two breast radiologists, B.K.S. and Y.W.O.
All images obtained, whether by CI, RTCI, or PIHI, were evaluated by three other radiologists (H.R.K., H.W.K., C.H.K.) in terms of lesion conspicuity and were further characterized according to a lesion margin, internal echotexture, and posterior echo pattern. Margins were classified as well-defined, ill-defined, microlobulated, spiculated, or angular, and wall-echo depiction was also evaluated. Internal echotexture was evaluated in terms of echo attenuation (hyper-, iso-, or hypoechogenicity), homogeneity, and internal microcalcification, and its clarity was also assessed. As for the posterior echo pattern, this was evaluated with regard to its attenuation (shadowing or enhancement) and clarity. Three radiologists graded image quality from 3 (the best) to 1 (the worst).
Friedman's test was used for multiple statistical comparison between the three techniques, and interobserver agreement in image interpretation was assessed using the intra-class correlation coefficient (ICC) (21) (SAS/STAT software, version 6.12; SAS Institute, Cary, NC).
ICCs were more than 0.44 in all four aspects of evaluation, and interobserver agreement was fair or excellent (Table 1).
Table 2 shows, for each of the three US techniques, the mean frequency with which each grade was assigned to each of these four evaluative parameters. With regard to lesion conspicuity, RTCI was judged significantly superior to both PIHI and CI, while PIHI was better than CI (p < 0.05) (Table 3) (Fig. 1).
The margin of the 52 nodules was well-defined in 35 cases (67%), ill-defined in six (11%), microlobulated in four (8%), angular in three (6%), spiculated in two (4%), angular and branching in one (2%), and microlobulated and spiculated in one (2%). In depicting the margin, RTCI was far superior to CI and PIHI, and PIHI was better than CI (p < 0.05) (Table 3) (Figs. 1, 2).
With regard to internal echotexture, attenuation was isoechoic in 22 cases (42%), hypoechoic in 18 (35%), hyperechoic in nine (17%), and anechoic with internal septation in three (6%). Thirty-four of the 52 nodules showed a homogeneous internal echotexture and the remaining 18 were heterogeneous. Internal microcalcification within the nodules was present in nine. In terms of clarity of the internal echotexture, RTCI was significantly superior to both PIHI and CI (p < 0.05) and PIHI was better than CI, though statistically, these two modalities were not significantly different (p > 0.05) (Table 3) (Figs. 2, 3, 4).
The most important roles of US in breast imaging include 1) the diagnosis of cysts, 2) the characterization of a) masses that have been incompletely assessed by mammography and b) palpable masses that are obscured by dense tissue at mammography, and 3) the provision of imaging guidance for percutaneous biopsy and localization. On the basis of breast US finding, it is, however, often difficult to differentiate benign from malignant nodules. In addition, the accuracy of this modality depends on operator skill and experience and it is time consuming.
US techniques such as harmonic and compound imaging have recently been introduced. The former has two modes, tissue harmonic imaging and PIHI. In tissue harmonic imaging, the US beam transmits at one frequency and receives at twice that frequency, that is, the second harmonic frequency. A bandpass filter is used to process the received signal so that only the returning high-frequency harmonic signal is used to produce the image. Resolution and sensitivity are thus limited by a fundamental compromise in the frequency-filtering approach (22). Instead of frequency filtering, PIHI transmits multiple identical pulses with reversed polarity; adding these resultant returned signals cancels the fundamental linear components and preserves the nonlinear harmonic components. Since no filtering technique is used, this procedure uses broader transmitting and receiving bandwidths and improves resolution. Furthermore, because of better deletion of the fundamental component for improved resolution, improved sensitivity is also achieved (23). In this study, PIHI was superior to CI in terms of lesion conspicuity and depiction of the margin of breast nodules, and this might be because of the high-contrast resolution of harmonic imaging. However, the overall echotexture of harmonic imaging is coarse compared to what is achieved by CI. In this study, there was no significant difference between CI and PIHI as regards the clarity of the internal echotexture of the nodules, and this may be related to the overall coarse echo pattern seen in harmonic imaging.
In studies involving the kidney and liver, the superior results of PIHI compared to tissue harmonic imaging showed close correspondence with these theoretical advantages (19, 20). Although PIHI provides significantly better image quality in the liver or kidney, it nonetheless suffers several limitations. Since multiple pulses are used, the frame rate is relatively low compared with CI, and since each ray line is formed individually, the technique is susceptible to motion artifacts. We used PIHI in this study because our US equipment has no tissue harmonic mode for linear probing, only PIHI.
The application of compounding principles to RTCI is not new (24, 25), but the practical implementation of this technology has only recently been made possible by the substantial computational power of modern, all-digital ultrasound systems. RTCI starts by acquiring multiple frames from different viewing angles; the overlapping frames are then combined to form an real-time compound image on the display. Compound images can be obtained using a conventional imager, with two modifications. First, the ultrasound beams are steered 'off-axis' from the 90° beams used in CI. Second, the image processor must be programmed to accurately render the steered frames into the appropriate display geometry, and then combine them through frame averaging. This, however, introduces a persistence effect, with the potential for image blurring if the transducer or the target moves too rapidly. The ability of RTCI to improve image quality depends primarily upon the suppression of image artifacts. An in-vitro study by Jespersen et al. (26), who quantitatively investigated the speckle reduction effect associated with compound imaging, demonstrated that multi-angle compound imaging reduced speckle artifacts in cross-sectional images of rubber tube phantoms and porcine aorta. Reduced speckle also contributes to better definition of boundaries and better detection of low-contrast regions or echo-strong microstructures such as microcalcifications. Speckle may be described as a granular effect visible in an otherwise homogeneous region of breast parenchyma. It reduces image contrast and detail resolution and diminishes the ability to differentiate normal tissue from lesion within the breast. In this study, RTCI significantly improved lesion conspicuity, depiction of the margin, and the clarity of internal echotexture of breast nodules when compared to CI, differences which might be caused by speckle reduction in compound imaging. Clutter, which consists of spurious echoes which can often be visualized within a breast cyst and may lead to concern as to whether a cyst is simple or complex, arises from side lobes or granting lobes, or because of multi-path reverberation. In RTCI, scanning from different angles produces different artifact patterns, and the averaging of these independent frames suppresses the artifacts and reinforces real structures.
In RTCI, however, the frame rate may be reduced. Our US equipment permits RTCI two modes, the survey mode providing three coplanar images, and the target mode, nine. In the former, the frame rate is the same as in CI, but in the target mode it is reduced. Thus, the survey mode minimizes blurring caused by motion artifact, and the target mode maximizes image quality through the use of many coplanar images. The survey mode is usually used in routine breast US for minimizing motion blurring caused by respiration, a probe, or a movable lesion, and for preservation of the frame rate.
The results of our study showed that in terms of lesion conspicuity and characterization, the two new techniques of RTCI and PIHI were superior to CI, a finding which shows close correspondence with the theoretical advantages. Improved lesion conspicuity and depiction of the margin may be attributed to the narrower dynamic range in PIHI and the reduction of artifacts such as speckle in RTCI. As for clarity of internal echotexture, RTCI was significantly superior to the other two techniques, which may be due to the compounding of multiple different frames and reinforcement of the nodules' real internal echo. PIHI provided a clearer internal echotexture than did CI, though the difference was not statistically significant; there may be a connection between this finding and the overall coarse echotexture of harmonic imaging. PIHI provided a clearer posterior echo pattern than the other two techniques, which might be related to the higher receiving frequency and narrower dynamic range used in harmonic imaging. Since higher frequency US is more attenuated when passing through echogenic areas, the difference in sonic attenuation between an echogenic area and one that is anechoic may increase and posterior echoes may be amplified. RTCI provided images whose posterior echo pattern was less clear than those provided by CI. In RTCI, multiple different steered angles are used, and posterior echoes are concentrated in a triangular region. Thus, posterior echoes are preserved in the central portion of the nodules and reduced in the peripheral portion, and overall are less frequent than in CI. We assume that after scanning with RTCI, it might be necessary to use PIHI or CI for evaluation of a nodule's posterior echo pattern.
Fifteen of the 52 nodules included in this study were examined pathologically. In one case of invasive ductal carcinoma, a neodensity was discovered at follow-up mammography and US was then performed. At CI, this nodule appeared slightly hypoechoic, but the type of margin, which at RTCI and PIHI was seen to be microlobulated and spiculated, with an internal echotexture that was significantly hypoechoic, could be determined, and malignancy was thus assumed (Fig. 1). In one case of atypical ductal hyperplasia, a well-defined oval-shaped isoechoic nodule, 10 mm in size and with posterior enhancement and lateral shadowing at CI was present. At RTCI its internal heterogeneous echotexture was emphasized, and at PIHI several internal microcalcifications were visible (Fig. 4). We believe this may have been a nodule which was shown by CI to be benign, but after RTCI and PIHI malignancy was suspected. We consider that these new US techniques might be useful in the detection and characterization of breast nodules, though in order to more fully assess their accuracy, further studies are needed.
In conclusion, RTCI and PIHI are better than CI in terms of lesion conspicuity and the characterization of breast nodules. RTCI is the best technique for evaluation of the margin and internal echotexture of nodules, and PIHI is very efficient for evaluation of their posterior echo pattern.
Figures and Tables
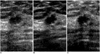 | Fig. 1Sonograms of a 56-year-old woman show an oval-shaped, microlobulated and spiculated marginated, hypoechoic nodule. In lesion conspicuity and margin depiction, real-time compound (B) and pulse-inversion harmonic imaging (C) are better than conventional scanning (A). Pathologic examination showed that the case was invasive ductal carcinoma. |
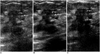 | Fig. 2Sonograms of a 46-year-old woman demonstrate an irregular-shaped, heterogeneously hypoechoic nodule with angular and branching margin. Internal microcalcifications (arrows) are seen within the nodule. Real-time compound imaging (B) is the best technique for evaluation of the margin and internal echotexture, compared to conventional (A) and pluse-inversion harmonic imaging (B). Pathologic examination indicated the presence of invasive ductal carcinoma. |
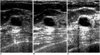 | Fig. 3Sonograms of a 35-year-old woman depict a septated cyst. The internal transverse thin septum within the cyst is more clearly demonstrated by real-time compound (B) and pulse-inversion harmonic imaging (C) than by conventional scanning (A). |
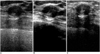 | Fig. 4Sonograms of 32-year-old woman reveal a well-defined, oval-shaped, heterogeneous isoechoic nodule. The heterogeneous internal echotexture is more clearly visualized by real-time compound imaging (B) than conventional (A) and pulse-inversion harmonic imaging (B) and an internal microcalcification (curved arrow) is revealed by pulse-inversion harmonic imaging (C). Pathologic examination showed that the condition was atypical ductal hyperplasia. |
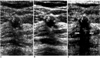 | Fig. 5Sonograms of a 52-year-old woman show a well-defined, hypoechoic, round nodule with internal microcalcifications. Posterior shadowing (arrows) due to microcalcifications is more clearly visualized at pulse-inversion harmonic imaging (C) than conventional (A) and real-time compound imaging (B). Pathologic examination indicated the presence of degenerating fibroadenoma. |
References
1. Hilton SW, Leopold GR, Olson LK, Wilson SA. Real-time breast sonography: application in 300 consecutive patients. AJR. 1986. 147:479–486.
2. Cole-Beuglet C, Soriano RZ, Kurtz B, et al. Ultrasound analysis of 104 primary breast carcinomas classified according to histopathologic type. Radiology. 1983. 147:191–196.
3. Cole-Beuglet C, Soriano RZ, Kurtz AB, Goldberg BB. Fibroadenoma of the breast: sonomammography correlated with pathology in 122 patients. AJR. 1983. 140:369–375.
4. Jackson VP, Rothschild PA, Kreipke DL, et al. The spectrum of sonographic findings of fibroadenoma of the breast. Invest Radiol. 1986. 21:34–40.
5. Heywang SH, Lipsit ER, Glassman LM, et al. Specificity of ultrasonography in the diagnosis of benign breast masses. J Ultrasound Med. 1984. 3:453–461.
6. Jokich PM, Monticciolo DL, Adler YT. Breast ultrasonography. Radiol Clin North Am. 1992. 30:993–1009.
7. Kopans DB, Meyer JE, Lindfors KK, Bucchianeri SS. Breast sonography to guide cyst aspiration and wire localization of occult solid lesions. AJR. 1984. 143:489–492.
8. Kopans DB. Breast imaging and the standard of care for the symptomatic patient. Radiology. 1991. 187:608–611.
9. Fornage BD, Sneige N, Faroux MJ, Andry E. Sonographic appearance and ultrasound guided fine-needle aspiration biopsy of breast carcinomas smaller than 1cm3. J Ultrasound Med. 1990. 9:559–560.
10. Fornage BD, Lorigan JG, Andry E. Fibroadenoma of the breast: sonographic appearance. Radiology. 1989. 172:671–675.
11. Harper PA, Kelly-Fry E, Noe JS, Bies RJ, Jackson VP. Ultrasound in the evaluation of solid breast masses. Radiology. 1983. 146:731–736.
12. Kobayashi T. Diagnostic ultrasound in breast cancer: analysis of retrotumorous echo patterns correlated with sonic attenuation by cancerous connective tissue. J Clin Ultrasound. 1979. 7:471–479.
13. Leucht WJ, Rabe DR, Humbert KD. Diagnostic value of different interpretative criteria in real-time sonography of the breast. Ultrasound Med Biol. 1988. 14:S 1. 59–73.
14. Majewski A, Rosenthal H, Wagner HH. Results of real-time sonography and raster mammography of 200 breast cancers. ROFO Fortschr Nucleamed. 1986. 144:343–350.
15. Smallwood JA, Guyer P, Dewbury K, Mengatti S, Royle GT, Taylor I. The accuracy of ultrasound in the diagnosis of breast disease. Ann R Coll Surg Engl. 1986. 68:19–22.
16. Ueno E, Tohno E, Soeda S, et al. Dynamic tests in real-time breast echography. Ultrasound Med Biol. 1988. 14:S 1. 53–57.
17. Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995. 196:123–134.
18. Shapiro RS, Wagreich J, Parsons RB, Stancato-Pasik A, Yeh HC, Lao R. Tissue harmonic imaging sonography: evaluation of image quality compared with conventional sonography. AJR. 1998. 171:1203–1206.
19. Jang HJ, Lim HK, Lee WJ, et al. Ultrasonographic evaluation of focal hepatic lesions: comparison of pulse inversion harmonic, tissue harmonic, and conventional imaging techniques. J Ultrasound Med. 2000. 19:293–299.
20. Kim BH, Lim HK, Choi MH, et al. Detection of parenchymal abnormalities in acute pyelonephritis by pulse inversion harmonic imaging with or without microbubble ultrasonographic contrast agent: correlation with computed tomography. J Ultrasound Med. 2001. 20:5–14.
21. Shrout PE, Fleiss Jl. Intraclass correlations: used in assessing rater reliability. Psychol Bull. 1979. 86:420–428.
22. Kim AY, Kim TK, Kim YH, Han JK, Choi BI. Comparison of harmonic and conventional power Doppler ultrasonography for the assessment of slow flow in hyperechoic tissue: experimental study using a Doppler phantom. Invest Radiol. 2000. 35:105–110.
23. Burns PN, Wilson SR, Simpson DH. Pulse inversion imaging of liver blood flow: improved method for characterizing focal masses with microbubble contrast. Invest Radiol. 2000. 35:58–71.
24. Carpenter DA, Dadd MJ, Kossoff FG. A multi-mode real-time scanner. Ultrasound Med Biol. 1980. 6:279–284.
25. Berson M, Roncin A, Pourcelot L. Compound scanning with an electrically steered beam. Ultrasonic Imaging. 1981. 3:303–308.
26. Jespersen SK, Wilhjelm JE, Sillesen H. Multi-angle compound imaging. Ultrasonic Imaging. 1998. 20:81–102.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download