Abstract
Objective
To determine the helical CT findings which help differentiate between focal eosinophilic necrosis (FEN) of the liver and metastasis in patients with underlying gastric or colorectal cancer.
Materials and Methods
In 21 patients with underlying gastric and colorectal cancer examined during a recent 18-month period, the presence of FEN (n=90) was proven at CT. The diagnosis was verified by biopsy in eight patients and by the transient nature of the findings related to peripheral eosinophilia (>10%) in the remainder. For comparison, 20 consecutive patients with pathologically proven hepatic metastasis from gastric or colorectal cancer (n=158) were selected. Single-phase helical CT images (7-mm collimation, pitch 1:1) were independently analyzed in a random order by two blinded readers. The parameters evaluated included the margin (depicted border, fuzzy), shape (spherical, non-spherical), attenuation (subtle hypoattenuation, hypoattenuation), and the presence or absence of rim enhancement.
Results
FEN far more frequently showed a fuzzy margin (81%, 84%), subtle hypoattenuation (89%, 91%), and a non-spherical shape (84% for both readers) than metastasis, for which the respective findings were 6%, 22%; 20%, 39%; and 15%, 23%. Rim enhancement was seldom found in FEN (0%, 2%), but was recognized by both readers in 40% of metastases. For all parameters, the results were statistically significant (p < .01), and showed that both readers correctly differentiated FEN from metastasis in 78% of the patients (32/41). Interobserver agreement was, in addition, excellent (κ= 0.66).
Focal eosinophilic necrosis (FEN) of the liver is one of various focal hepatic lesions caused by eosinophil-related tissue damage (1). Since eosinophilia can occur in a variety of pathologic conditions such as allergic reactions, parasitic infestations, connective tissue disorders and neoplastic diseases, FEN comprises a number of heterogeneous eosinophilia-related conditions. Although the entity has not been clearly defined and the mechanisms are not fully understood, FEN has recently received much attention. It is mentioned in imaging studies far more frequently than in the past, and is consequently often confused with other focal hepatic lesions. FEN is particularly problematic when found in patients with underlying malignancy. At preoperative CT it is apt to be misinterpreted as metastasis, especially when first appearing during follow-up, and in such patients, correct interpretation of radiologic indications that FEN may exist is critical to proper management. Ultrasound (US) can be used to confirm the presence of cysts (2, 3), but not of FEN, and for this reason, a way of characterizing the latter on the basis of CT findings is needed. Hence, we performed a comparative study to determine the helical CT features which help distinguish FEN from metastasis in patients with underlying gastric and colorectal cancer.
Using the radiologic information system, we searched our departmental medical records dated November 1998 to April 2000 for patients whose final diagnosis was gastric or colorectal cancer and who had also undergone contrast-enhanced abdominal and pelvic CT using the protocol employed for the liver. We identified 21 consecutive patients [M : F=15 : 6; age, 31-72 (mean, 54) years] with underlying gastric or colorectal cancer in whom the presence of FEN (n=90) was also proven, and these formed the study population. In eight patients, the diagnosis of FEN (1) was verified by percutaneous or intraoperative biopsy, and in the remainder by consistent clinical findings of associated peripheral eosinophilia (> 10%) followed by its spontaneous disappearance at CT after the peripheral blood eosinophil count normalized. To avoid the possibility of coincidental metastasis and eosinophilia, patients who underwent chemotherapy during the follow-up period were excluded. The peripheral eosinophil count was normal in two of eight patients with pathologically proven FEN, ranging from 0.4 to 21.7% (mean, 13.2%). Eighty-two FENs in 18 patients were detected at initial preoperative CT and the remaining eight, in three patients, were new and detected during the follow-up period.
For the purpose of comparison, and using records covering the same period, 20 consecutive patients with pathologically proven hepatic metastasis (n=158) from gastric (n=101) or colorectal cancer (n=57) were also selected.
In all cases, CT examinations were performed with a helical CT scanner (HiSpeed Advantage; General Electric Medical Systems, Milwaukee, Wis., U.S.A.) after the intravenous administration of 120 mL of nonionic contrast material (Ultravist 300; Schering AG, Berlin, Germany) at a rate of 2.5 mL/sec and with an injection delay of 70 seconds. For patients with gastric cancer, helical mode scans were obtained from the level of the hepatic dome to that of the renal hilum, with patients in the prone position, and with 7-mm collimation, a pitch of 1, and 7-mm reconstruction interval. These patients drank 500-800 mL of water 50-60 minutes before scanning and an additional 500 mL just before scanning. Those with colorectal cancer underwent scanning of the upper abdomen from the level of the hepatic dome to the inferior tip of the liver, using the same helical mode used for gastric cancer patients, and in the supine position. For scanning of the rest of the abdomen and pelvis, the clustered data acquisition mode (5-mm collimation and 5-mm interval) was used. Patients drank 600-900 mL of 2.5% diluted sodium amidotrizoate and meglumine amidotrizoate mixture (Gastrografin, Schering, Germany) 50-60 minutes before CT scanning, and water was administered rectally.
Two experienced abdominal radiologists with no knowledge of the final diagnoses independently analyzed single-phase helical CT images of both FEN and metastasis in a random order. The parameters evaluated included the margin, attenuation, shape, and the presence or absence of rim enhancement. The window settings of all CT images were fixed at a width of 250 and a level of 30. The margin was categorized as 'depicted border' or 'fuzzy', depending on whether or not a complete margin could be drawn. Attenuation was classified as either 'subtle hypoattenuation' (discernibly lower than that of hepatic parenchyma) or 'hypoattenuation' (higher than that of bile in the gallbladder and lower than 'subtle hypoattenuation'), and shape as either spherical or non-spherical. The presence or absence of a rim enhancement pattern (hypoattenuation at the center surrounded circumferentially by a less hypoattenuating border) was determined. For statistical analysis of each parameter, the chi-square test or Fisher's exact test was used, and degree of interobserver agreement was expressed by means of a kappa statistic; as suggested by Landis and Koch (4), a κ value greater than 0.60 indicated excellent agreement, between 0.40 and 0.60 was good, and less than 0.40 was poor.
The results of image analysis are summarized in Table 1.
FEN (Fig. 1) far more frequently showed a fuzzy margin (81%, 84%), subtle hypoattenuation (89%, 91%), and non-spherical shape (85%, 84%) than metastasis, for which the corresponding figures were 6%, 22%; 20%, 39%; and 15%, 23%. Rim enhancement was seldom found in FEN (0%, 2%), whereas it was recognized by both readers in 40% of cases involving metastasis. For all four parameters, these results were statistically significant (p < .01), and there was also excellent interobserver agreement (κ > 0.60). Both readers correctly differentiated FEN from metastasis in 78% of patients (32/41).
Although 'focal eosinophilic necrosis' is a descriptive term based on pathologic features and has not yet been clearly defined, its imaging findings have lately attracted considerable attention because in daily practice the condition often gives rise to diagnostic dilemmas. Most radiologic reports, however, have focused on hypereosinophilic syndrome (5-9), a clinically distinct entity from FEN affecting patients with mild eosinophilia (1) such as those described in this study. As FEN is in most cases detected incidentally and usually manifests at CT as multiple hypoattenuating lesions, differentiation from metastasis has been a problem, particularly in patients with underlying extrahepatic malignancies.
At multiphasic helical CT, FEN is generally most conspicuous during the portal venous phase (Fig. 2) but is often not visualized during the hepatic arterial or equilibrium phase (1, 5), and this may be why it was seldom an issue in the era of conventional CT. Owing to the optimal hepatic parenchymal enhancement achieved by helical CT (10), the incidence of FEN at imaging studies appears to be increasing, giving rise to a clinical problem. Since single, portal phase CT is generally performed for routine work-up for metastasis or follow-up, characterization of FEN and its differentiation from metastasis on the basis of single-phase CT findings is needed.
Our results showed that FENs were far more likely to have a fuzzy margin, and be non-spherical in shape and subtly hypoattenuating. In addition, it is noteworthy that rim enhancement was seen almost exclusively in metastasis and seldom - in up to only 2% of cases - in FEN. Because its incidence in cases involving metastasis is low (40%), and it may thus not be a good indicator of this, rim enhancement could be a helpful feature in discriminating metastasis from FEN. Metastatic nodules tend to outgrow their blood supply, producing central necrosis and umbilication (11), and these features could be represented as target or rim enhancement. On the other hand, FEN is usually unaccompanied by a peripheral collection of histiocytes or fibroblasts, as seen in granulomas or abscesses, that could produce peripheral enhancement. In pathologic terms, FEN is basically a focal area of heptaocellular necrosis caused by severe eosinophilic infiltration of the perivascular space (1), and this may explain the frequently-noted irregular shape and fuzzy margins.
Among the 90 FENs in our study, 82 lesions were detected at initial preoperative CT and the remaining eight were new, and detected during the follow-up period. Where small indeterminate hepatic lesions occur, an absence of change compared with prior imaging findings is generally recognized as characteristic of benignancy (12); conversely, new focal hepatic lesions found during follow-up in oncologic patients have usually been considered metastatic (Fig. 3). Radiologists are thus perplexed to find that in such patients, a new hepatic nodule could be due to a benign transient condition such as FEN, and familiarity with the CT findings of FEN and radiologic suggestion of the disease may play a pivotal role in its proper management.
In our experience, FEN seems to almost always be accompanied by peripheral eosinophilia. However, the peripheral eosinophil count was normal in two of our eight patients with pathologically-proven FEN, and it may thus be assumed that among indeterminate lesions classified as 'non-FEN' due to a lack of peripheral eosinophilia, FEN may be actually present. Even in oncologic patients without eosinophilia, when indeterminate hepatic lesions show CT findings consistent with FEN, biopsy should be performed before deciding that they are metastatic (Table 2).
Malignancies such as lymphoma, leukemia and carcinoma are often reported to be associated with eosinophilia; hence, eosinophil-related hepatic damage, such as the FEN described in our study, may arise. One report noted the occurrence of this phenomenon in lymphoma cases involving the tumor-associated eosinophilotactic factor, which is identical to the eosinophil-chemotactic factor of anaphylaxis (13). In our experience, FEN not infrequently occurs in patients with gastrointestinal malignancies, and might also be due to factors derived from the tumors themselves; because FEN could, therefore, indicate the presence of tumor foci at other sites, care must be exercised when FENs are observed during follow-up.
Our study suffers certain limitations. First, not all FENs were pathologically proven. Partly because familiarity with the CT findings of FEN has reduced the need for further invasive study, and partly because FEN is not clearly visible at US, biopsy was not always performed. Although we confirmed the benign nature of the lesions by observing their complete resolution at follow-up CT and by excluding patients who underwent chemotherapy during the follow-up period, we still cannot be absolutely certain that no other transient benign conditions existed. Second, our study included only patients with gastric or colorectal cancers, and whether these results can be generally applied to patients with various types of malignancy is thus open to debate.
In summary, when focal hepatic lesions have a fuzzy margin, are non-spherical in shape, and show homogeneous subtle hypoattenuation without rim enhancement, the possibility of FEN should be considered even in patients with underlying gastrointestinal malignancy.
Figures and Tables
Fig. 1
Focal eosinophilic necrosis of the liver in a 51-year-old man with gastric cancer and peripheral eosinophilia (11.7%). Contrast-enhanced CT scan obtained during the portal venous phase shows that in both hepatic lobes, several non-spherical lesions (arrows) with a fuzzy margin are present, and there is subtle hypoattenuation without rim enhancement.
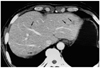
Fig. 2
Contrast-enhanced helical CT scan of focal eosinophilic necrosis of the liver in a 49-year-old man with colon cancer and peripheral eosinophilia (13.1%).
A. Hepatic arterial-phase image depicts a minimally conspicuous but very suspicious lesion (arrow) in the right hepatic lobe.
B. Portal venous-phase image reveals greater lesion conspicuity (straight arrow) than is apparent in A. Also visible are two additional lesions (curved arrows).
C. Image obtained after a three-minute delay depicts no recognizable lesions.
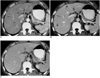
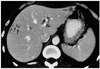
Fig. 3
Metastases from rectal cancer first evident at follow-up CT performed seven months after surgery in a 62-year-old man. Contrast-enhanced helical CT scan obtained during the portal venous phase shows small hypoattenuating lesions (arrows) with an obvious rim- or target-like enhancement pattern.
References
1. Lee WJ, Lim HK, Lim JH, Kim SH, Choi SH, Lee SJ. Foci of eosinophil-related necrosis in the liver: imaging findings and correlation with eosinophilia. AJR Am J Roentgenol. 1999. 172:1255–1261.
2. Brick SH, Hill MC, Lande IM. The mistaken or indeterminate CT diagnosis of hepatic metastasis: the value of sonography. AJR Am J Roentgenol. 1987. 148:723–726.
3. Gaines PA, Sampson MA. The prevalence and characterization of simple hepatic cysts by ultrasound examination. Br J Radiol. 1989. 62:335–337.
4. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977. 33:159–174.
5. Cha SH, Park CM, Cha IH, et al. Hepatic involvement in hypereosinophilic syndrome: value of portal venous phase imaging. Abdom Imaging. 1998. 23:154–157.
6. Lim JH, Lee WJ, Lee DH, Nam KJ. Hypereosinophilic syndrome: CT findings in patients with hepatic lobar or segmental involvement. Korean J Radiol. 2000. 1:98–103.
7. Fauci AS, Harley JB, Robert WC, Ferrans VJ, Gralnick HR, Bjornson BH. The idiopathic hypereosinophilic syndrome: clinical, pathophysiologic, and therapeutic considerations. Ann Intern Med. 1982. 97:78–92.
8. Kim GB, Kwon JH, Kang DS. Hypereosinophilic syndrome: imaging findings in patients with hepatic involvement. AJR Am J Roentgenol. 1993. 161:577–580.
9. Nam KJ, Jung WJ, Choi J-C, et al. Hepatic involvement in hypereosinophilia: sonographic findings. J Ultrasound Med. 1999. 18:475–479.
10. Oliver JH III, Baron RL. Helical biphasic contrast-enhanced CT of the liver: technique, indications, interpretation, and pitfalls. Radiology. 1996. 201:1–14.
11. Cotran RS, Kumar V, Robbins SL. Robbins pathologic basis of disease. 1989. 4th ed. Philadelphia: Saunders;962.
12. Schwartz LH, Gandras EJ, Colangelo SM, Ercolani MC, Panicek DM. Prevalence and importance of small hepatic lesions found at CT in patients with cancer. Radiology. 1999. 210:71–74.
13. Wasserman SI, Goetzl EJ, Ellman L, Austen KF. Tumor-associated eosinophilotactic factor. N Engl J Med. 1974. 21:420–424.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download