Abstract
Objective
To compare the age distribution and characteristic MR imaging findings of ependymoma for each typical location within the neuraxis.
Materials and Methods
During a recent eleven-year period, MR images of 61 patients with histologically proven ependymomas were obtained and retrospectively reviewed in terms of incidence, peak age, location, size, signal intensity, the presence or absence of cyst and hemorrhage, enhancement pattern, and other associated findings.
Results
Among the 61 patients, tumor location was spinal in 35 (57%), infrartentorial in 19 (31%), and supratentorial in seven (12%). In four of these seven, the tumor was located in brain parenchyma, and in most cases developed between the third and fifth decade. Approximately half of the infratentorial tumors occurred during the first decade. The signal intensity of ependymomas was non-specific, regardless of their location. A cystic component was seen in 71% (5/7) of supratentorial, 74% (14/19) of infratentorial, and 14% (5/35) of spinal cord tumors. Forty-nine percent (17/35) of those in the spinal cord were associated with rostral and/or caudal reactive cysts. Intratumoral hemorrhage occurred in 57% (4/7) of supratentorial, 32% (6/19) of infratentorial, and 9% (3/35) of spinal cord tumors. In 17% (6/35) of spinal ependymomas, a curvilinear low T2 signal, suggesting marginal hemorrhage, was seen at the upper and/or lower margins of the tumors. Peritumoral edema occurred in 57% (4/7) of supratentorial, 16% (3/19) of infratentorial and 23% (8/35) of spinal cord tumors. Seventy-two percent (5/7) of supratentorial and 95% (18/19) of infratentorial tumors showed heterogeneous enhancement, while in 50% (17/34) of spinal cord tumors, enhancement was homogeneous.
Ependymomas are glial tumors derived from differentiated ependymal cells lining the ventricles of the brain and the central canal of the spinal cord (1). They are rare neoplasms of the central nervous system (CNS), accounting for approximately 2-9% of intracranial gliomas and 30-60% of intramedullary spinal tumors (2). A majority occur infratentorially, arising from the floor of the fourth ventricle (3), while most others arise from the outer wall of the lateral ventricles or within the spinal canal.
These tumors have a variety of imaging characteristics, some of which are fairly specific. Most previous reports of ependymomas have described their radiologic findings according to the site of origin, though a few have addressed the differences in MR imaging features, if any, found in a series of intracranial and spinal cord tumors according to location (4). The purpose of this study is to review the overall MR imaging findings of ependymomas found in various locations within the neuraxis, and for each typical location, to compare the age distribution and characteristic features.
We retrospectively reviewed the initial MR images, obtained at our institution between January 1990 and January 2001, of 61 patients [M:F=39:22; age at presentation, 1-70 (mean, 29.1) years] with proven primary ependymoma. For MR examinations, 0.5-, 1.0-, or 1.5-T units, produced by various manufacturers, were employed. Images were usually obtained using axial T2-weighted conventional or fast spin-echo sequences (TR/TE=4000/98) and sagittal T1-weighted sequences (TR/TE=450/10). Sixty patients underwent enhanced spin-echo T1-weighted imaging in the axial and sagittal planes after the injection of 0.1 mmol/kg gadopentetate dimeglumine (Magnevist; Schering, Berlin, Germany).
Three observers reviewed all MR images, reaching a consensus. Tumor location was categorized as supratentorial, infratentorial, or spinal, the last of these being subcategorized as cervical, thoracic, or filar. If a tumor extended across two or more regions, it was classified according to the site of its greatest mass. At each location, the position of a tumor was determined. Supratentorial ependymomas were classed as transependymal if they arose within the ventricular system but breached the ependymal lining to invade surrounding parenchyma, or as intraventricular or intraparenchynal. Infratentorial tumors were either intraventricular, or were associated with extension from the fourth ventricle through the foramen of Magendie or Luschka. A spinal tumor was considered central if axial imaging showed that no more than 60% of its cross-sectional area was located to one side of the midline, or eccentric if this was not the case (5). The size of an intracranial lesion was determined according to its longest diameter, and for a spinal location, according to the number of spinal segments involved.
For each imaging sequences, the signal intensity of an intracranial and a spinal tumor was compared, respectively, with that of gray matter and spinal cord.
A tumor was considered to have a cystic component if a cyst associated with it contained an enhanced nodule or had an enhanced wall. Especially in spinal cord ependymomas, this type of tumor is in contradistinction to the nonenhanced, non-neoplastic rostral and caudal reactive cysts often associated with spinal cord tumors. The distinction between rostral /caudal cysts and reactive dilatation of the central canal can be difficult, and secondary reactive dilatation of this kind was classified as a cyst (6). If areas of high signal intensity were obscured at non-enhanced T1-weighted imaging, intratumoral hemorrhage was thought to be present. In ependymomas of the spinal cord, the presence or absence of a low signal intensity rim at the boundaries of the tumor at T2-weighted imaging, which suggested marginal hemorrhaging, was also evaluated.
Peritumoral edema was scored as absent, mild (affecting an area less than half that of the tumor), moderate (affecting an area greater than half that of tumor, but not greater than that of the tumor itself), or marked (affecting an area greater than that of the tumor).
The presence and pattern of tumor enhancement and the definition of enhanced borders were assessed. The enhancement pattern was qualitatively evaluated as either homo- or heterogeneous, and enhanced margins as either sharply or poorly defined.
Of 61 ependymomas, seven (12%) were supratentorial, 19 (31%) were infratentorial, and 35 tumors (57%) were spinal.
Most supratentorial ependymomas (in six of seven patients) occurred in adults between their third and fifth decade. Ten infratentorial tumors (53%), on the other hand, developed during the first decade. Spinal cord tumors were found among all age groups, with two-thirds occurring in these between their third and fifth decade. The findings appear in Table 1.
The overall male to female ratio was 1.8:1 (39:22). Four (57%) of seven patients with supratentorial tumors, 12 (63%) of 19 with infratentorial tumors, and 23 (66%) of 35 with spinal cord tumors were male.
Of seven supratentorial ependymomas, one was entirely intraventricular, four were entirely intraparenchymal (Fig. 1), and two were transependymal (Fig. 2). Of 19 infratentorial tumors, only one was confined within the fourth ventricle. Sixteen (84%) extended caudally via the foramen of Magendie (Fig. 3), and 12 (63%) into the cerebellopontine angle through the lateral recesses and the foramen of Luschka. Ten extended both caudally and laterally via both the foramina of Magendie and Luschka (Fig. 4). Of 35 spinal cord tumors, 14 (40%) were cervical, 10 (29%) were thoracic, and 11 (31%) were filar in location. Transaxial images showed that the epicenters of 32 (91%) of these 35 tumors corresponded to the center of the spinal cord.
The longest diameter of supratentorial tumors ranged from 2.5 to 6.0 (mean, 4.5) cm; for intraparenchymal and intraventricular tumors, respectively, this measurement was 5.0 and 3.8 cm. Infratentorial tumors ranged in size from 3.0 to 7.0 (mean, 4.9) cm, and the extent of spinal cord tumors, measured along their neuraxis, was 1-13 (mean, 3.1) vertebral segments.
At TI-weighted imaging, three (43%) of seven supratentorial tumors were isointense relative to gray matter, and three others (43%) were hypointense (Fig. 2B); 11 (58%) of 19 infratentorial tumors and 22 (63%) of 35 spinal cord tumors were isointense. At T2-weighted imaging, six (96%) of seven supratentorial, 17 (89%) of 19 infratentorial and 33 (94%) of 35 spinal cord tumors were hyperintense relative to the signal intensity of gray matter. The others were isointense.
Five (71%) of seven supratentorial, 14 (74%) of 19 infratentorial, and five (14%) of 35 spinal cord ependymomas had an intratumoral cystic component (Fig. 4). Four of the five supratentorial tumors with this component were intraparenchymal (Fig. 1), and in 17 (49%) of 35 spinal cord tumors, rostral (n=16) and/or caudal (n=14) reactive cysts were present. Among the 14 cervical cord tumors, cyst type was rostral (n=9), caudal (n=10), or both (n=9) (Fig. 5), while the ten thoracic cord tumors contained either rostral (n=5), caudal (n=3), or both types of cyst (n=3). The cysts associated with the 11 filar tumors were either rostral (n=2), caudal (n=1) or of both types (n=1).
Intratumoral hemorrhage occurred in 13 (21%) of the 61 ependymomas [in four (57%) of seven supratentorial, six (32%) of 19 infratentorial, and three (9%) of 35 spinal cord tumors] (Figs. 1 and 4). At T2-weighted imaging, rim(s) of low signal intensity, suggesting marginal hemorrhage between normal and tumor tissue, were seen at the upper or lower margins of six (17%) of 35 spinal cord ependymomas; four of the six were cervical (Fig. 7), one was thoracic (Fig. 6), and one was filar.
Peritumoral edema occurred in four (57%) of seven supratentorial, three (16%) of 19 infratentorial, and eight (23%) of 35 spinal cord tumors. Among supratentorial tumors the edema was mild in two cases (Fig. 1), moderate in one, and severe in one, three (75%) of four intraparenchymal supratentorial tumors showed no or mild edema. In all three infratentorial tumors the edema was mild, and among spinal cord tumors it was mild in six and moderate in two (Fig. 7).
Among the 60 patients who underwent contrast-enhanced scanning, tumor enhancement was demonstrated in 56 patients (93%); in 53 (95%) of these (five of six supratentorial, 18 of 19 infratentorial, 30 of 31 spinal cord tumors), the enhanced margins were sharply defined. Enhancement was heterogeneous in five (71%) of seven supratentorial tumors and 18 (95%) of 19 infratentorial tumors (Fig. 2C), while among 34 spinal cord tumors, the observed pattern was homogeneous in 17 cases (50%) (Fig. 5) and heterogeneous in 14 (41%) (Fig. 6). In three cases (9%) there was no enhancement.
Ependymomas develop most often in children, adolescents and young adults, but may also occur in older age groups (3). In our series, most supratentorial ependymomas developed during the third to fifth decade, while approximately 50% of infratentorial ependymomas occurred during the first decade. Spinal cord tumors occurred between the first and seventh decade, with approximately two-thirds developing between the third and fifth. Although no significant difference in the frequency of ependymomas between the two genders has been reported (7), in our series they occurred 1.8 times more frequently in males than in females.
Although ependymomas are classically considered to occur most frequently in the fourth ventricle - infratentorial region (8), the most common location in our series was the spinal cord (57% of 60 lesions), followed by infratentorial and then supratentorial. Of all intracranial ependymomas, approximately 60% are infratentorial and 40% are supratentorial (9), though in our series, the ratio was approximately 7:3. Whereas supratentorial tumors tend to be parenchymal, infratentorial tumors are usually intraventricular (10), and we observed similar findings. The extraventricular location of most supratentorial ependymomas has been attributed to their origin in extraventricular ependymal cell rests (11), which are reported to occur mainly in regions where subependymal neural glia extend into adjacent white matter, e.g. at the angled margins of the ventricles (12). All four supratentorial intraparenchymal tumors in our series were large (average diameter, 5.0 cm), and focally abutted the adjacent ventricle. Thus, these imaging features may help differentiate between ependymoma and other supratentorial tumors.
"Desmoplastic development" is the term used to describe the way in which infratentorial ependymomas extend through the outlet foramina of the fourth ventricle into the subarachnoid space of the posterior cranial fossa. The prevalence of desmoplastic development through the foramen of Magendie and/or Luschka in our study was similar to that previously reported (13).
The signal intensity of ependymomas is nonspecific. In our study, regardless of their location within the neuraxis, they were hypo- to isointense to gray matter at non-enhanced T1-weighted imaging, and hyperintense at T2-weighted imaging, findings which were similar to those of other CNS tumors (14, 15).
Supratentorial ependymomas are commonly cystic (between 40 and 85%) (10-12, 16), and in our series, 71% (5/7), including all that were supratentorial, had cystic components. Our results confirm the observation of Furie and Provenzale (17), who reported that supratentorial extraventricular ependymomas are typically large cystic masses. As for infratentorial ependymomas, Tortori-Donati et al. (13) reported that MR imaging depicted cystic change in 65% of such cases; in our series, 74% (14/19) had cystic components. In contrast to intracranial ependymomas, these of the spinal cord are not usually cystic (between 4 and 50%) (5, 18); in our series, only 14% (5/35) had an intratumoral cystic component. Rostral and/or caudal cysts are frequently associated with intramedullary tumors of all histologic types (19). These cysts are important because they reflect a reactive process within the spinal cord, do not contain neoplastic cells, and do not need to be resected (19). However, the prevalence of associated reactive cysts does not serve as a distinguishing feature of ependymomas: Epstein et al. (20) reported that about 30% of tumors were associated with a rostral or caudal cyst similar to that noted in spinal cord astrocytoma cases. The frequency of associated cysts in our series (49%) was slightly higher.
Intratumoral hemorrhage occurs infrequently in ependymomas, with a prevalence of 0 to 13% in three reported series (5, 10, 21). In ours, the figure was slightly higher (21%), with regional variation (highest in supratentorial tumors, 57%; lowest in spinal cord tumors, 9%). At T2 imaging, the presence of a low-signal rim along the rostral or caudal margin of a spinal cord tumor is a fairly specific indicator of ependymoma, and was found in 20% and 64% of cases in the series investigated, respectively, by Fine et al. (5) and Nemoto et al. (22). The rim is due to the presence of hemosiderin at the margin of the tumor, arising, presumably, from prior subclinical tumoral hemorrhage, and is often referred to as a 'hemosiderin cap'. In our series, the 'cap' occurred slightly less frequently, in 17% of cases. It was more frequently observed in cervical tumors (29%), a finding similar to that of Fine et al. (5), and one which may support the idea that motional differences in neural tissues that occur at the relatively sharp interface between a tumor and the spinal cord partly account for the higher prevalence of marginal hemorrhage in the cervical region (22).
Where there is parenchymal involvement, peritumoral edema is usually mild. In our series, all infratentorial ependymomas, 94% of spinal cord ependymomas, and 71% of supratentorial ependymomas showed no or mild peritumoral edema. We believe that the relative lack of peritumoral edema in infratentorial and spinal tumors results from the space limitation imposed by their intraventricular or intraspinal location.
Most ependymomas in our series (93%) showed contrast enhancement, though the pattern of this varied. It was heterogeneous in 71% of supratentorial and 95% of infratentorial ependymomas, but homogeneous in 50% of spinal cord tumors. Parizel et al. (23) stated that spinal cord ependymomas generally show intense, homogeneous enhancement, while Fine et al. (5), on the other hand, reported heterogeneous enhancement in 65% (15/23) of such cases. In our study, five of 16 infratentorial ependymomas with caudal extension through the foramen of Magendie had an unenhanced intraventricular portion, whereas the caudally extended portion showed relatively intense enhancement. The margin of the enhanced area was sharply defined in 95% (53/56) of our cases. Fine et al. (5) stated that this finding is a characteristic feature of spinal cord ependymoma and is analogous to the surgical and histopathologic observation of a generally well-defined interface between ependymomas and adjacent spinal cord. Johnson et al. (24), however, emphasized that in intracranial tumors, isolated tumor-cell infiltration may exist outside the margin of contrast enhancement.
In summary, slightly more than half of the 61 ependymomas occurred in the spinal cord. Approxi-mately half the infratentorial ependymomas occurred during the first decade of age. Supratentorial ependymomas developed more frequently in brain parenchyma than in the ventricle. The signal intensity of an ependymoma is not specific, regardless of its location. The incidence of a cystic component was significantly higher in intracranial than in spinal ependymomas. The rate of focal intratumoral hemorrhage was higher in intracranial than in spinal tumors. A 'hemosiderin cap' was seen in 17% (6/35) of spinal ependymomas, most commonly in cervical tumors (4/6). Most ependymomas were accompanied by no or mild peritumoral edema, irrespective of their location. The observed pattern of contrast enhancement was more frequently heterogeneous in intracranial than in spinal ependymomas.
Figures and Tables
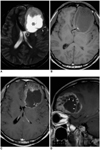 | Fig. 1A 21-year-old man with supratentorial intraparenchymal ependymoma.
A. Axial T2-weighted spin-echo (SE) image (4500/96) depicts a large tumor with an extensive cystic component (asterisk) in the left frontal lobe. The lesion focally abuts the adjacent frontal horn of the lateral ventricle (open arrow), and there is mild peritumoral edema (arrowheads).
B. Axial T1-weighted SE image (500/12) demonstrates an area of focal hyperintensity within the tumor (arrow),respresenting intratumoral hemorrhage. Due, presumably, to its high protein content, the cystic component appears isointense to gray matter.
C, D. Axial (C) and sagittal (D) contrast-enhanced T1-weighted SE images (500/12) show heterogeneous enhancement and a well-defined tumor margin. The cystic portion is multi-septated and enhanced (arrowheads).
|
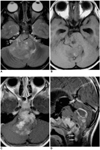
 | Fig. 2A 34-year-old man with supratentorial transependymal ependymoma.
A. Axial T2-weighted SE image (5000/99) depicts a slightly hyperintense, large tumor located within the frontal horns of both lateral ventricles (open arrow), which are dilated.
B. Axial T1-weighted SE image (600/12) shows that the tumor is slightly hypointense and has a lobulated margin.
C, D. Axial (C) and coronal (D) contrast-enhanced T1-weighted SE images (500/12) depict parenchymal invasion adjacent to the left frontal horn (arrowheads), with heterogeneous enhancement.
|
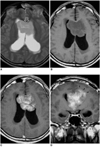
 | Fig. 3A 7-year-old boy with infratentorial ependymoma.
A, B. Axial T2-weighted SE image (A) (3000/100) and axial T1-weighted SE image (B) (650/25) demonstrate, respectively, hyper- and isointensity of the large tumor (arrows) seen at the midline of the posterior fossa. Associated mild peritumoral edema (arrowheads) is present.
C. Axial contrast-enhanced T1-weighted SE image (650/25) reveals heterogeneous enhancement and a cystic component.
D. Sagittal contrast-enhanced T1-weighted SE image (450/25) shows that the tumor extends caudally through the foramen of Magendie. Its intraventricular portion (asterisk) is not enhanced, whereas the caudally extended portion (in the cisterna magna) shows relatively intense enhancement (arrows).
|
 | Fig. 4A 3-year-old boy with infratentorial ependymoma.
A. Axial T2-weighted SE image (4500/96) depicts a large slightly hyperintense mass in the fourth ventricle. The mass extends to the right cerebellopontine angle and prepontine cistern through both lateral recesses and the foramen of Luschka (arrowheads), encasing a linear signal void (arrow) thought to be a vascular structure.
B. T1-weighted SE image (500/12) reveals the presence of a small area of focal high signal intensity (open arrow) within the mass, suggesting intratumoral hemorrhage.
C, D. Axial (C) and sagittal (D) contrast-enhanced T1-weighted SE images (500/12) show heterogeneous enhancement, with multiple cystic components and associated peripheral rim enhancement. The mass extends to the cerebellopontine angle bilaterally, and to the level of the upper cervical cord caudally (arrows).
|
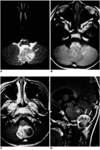
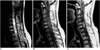 | Fig. 5A 30-year-old woman with spinal ependymoma of the cervical cord.
A, B. Sagittal T2-weighted SE image (A) (4000/120) shows that the mass at the level of C-5 to C-6 (arrows) is heterogeneously hyperintense, while T1-weighted image (B) (671/12) shows hypointensity. Associated rostral and caudal cysts are also visible.
C. Sagittal contrast-enhanced T1-weighted SE image (671/12) shows homogeneous enhancement and a well-defined, enhanced border (arrows). Extensive associated rostral and caudal cysts (asterisks) extend from the level of the foramen magnum to T-3.
|
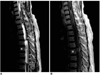 | Fig. 6A 50-year-old woman with spinal ependymoma at the thoracic level.
A. Sagittal T2-weighted SE image (3500/108) depicts an extensive heterogeneous lesion of mixed signal intensity in almost the entire spinal cord. Rostral and caudal cysts with inner multifocal fluid fluid levels (arrowheads) are extensive, and after previous hemorrhage, hemosiderin has been deposited. In addition, a dark line suggesting hemosiderin deposition (open arrow) is noted along the cord and the bottom of the caudal cyst. Asterisk indicates the T-5 level of the small enhancing tumor seen in B.
B. Sagittal contrast-enhanced T1-weighted SE image (500/14) depicts a small area of slightly heterogeneous enhancement at the T-5 level of the spinal cord (asterisk).
|
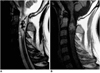 | Fig. 7A 27-year-old man with ependymoma of the cervical cord.
A. Sagittal T2-weighted SE image (3500/120) shows that in the upper cervical cord, a heterogeneously intense mass with a low signal intensity rim (arrows) at its upper and lower margins is present. Associated peritumoral edema (arrowheads) is also apparent.
B. Sagittal contrast-enhanced T1-weighted SE image (500/15) of the tumor depicts heterogeneous mild enhancement.
|
References
1. Russel DS, Rubenstein LJ. Pathology of tumors of the nervous system. 1989. 5th ed. Baltimore: Williams & Wilkins;192–219.
2. Rezai AR, Woo HH, Lee M, Cohen H, Zagzag D, Epstein FJ. Disseminated ependymomas of the central nervous system. J Neurosurg. 1996. 85:618–624.
3. Fokes EC, Earle KM. Ependymomas: clinical and pathological aspects. J Neurosurg. 1969. 30:585–594.
4. Lefton DR, Pinto RS, Martin SW. MRI features of intracranial and spinal ependymomas. Pediatr Neurosurg. 1998. 28:97–105.
5. Fine MJ, Kricheff II, Freed D, Epstein FJ. Spinal cord ependymomas: MR imaging features. Radiology. 1995. 197:655–658.
6. Kahan H, Sklar EM, Post MJ, Bruce JH. MR characteristics of histopathologic subtypes of spinal ependymoma. AJNR Am J Neuroradiol. 1996. 17:143–150.
7. Greenfield JG. Greenfield's Neuropathology. 1992. 6th ed. New York: Oxford University Press;636–644.
8. Barone BM, Elvidge AR. Ependymoma: a clinical survey. J Neurosurg. 1970. 33:428–438.
9. Kernhan JW, Sayre GP. Tumors of the central nervous system: Atlas of tumor pathology Section X, Fascicle 35. 1952. Washington: Armed Forces Institute of Pathology.
10. Armington WG, Osborn AG, Cubberley DA, et al. Supratentorial ependymoma: CT appearance. Radiology. 1985. 157:367–372.
11. Swartz JD, Zimmerman RA, Bilaniuk LT. Computed tomography of intracranial ependymomas. Radiology. 1982. 143:97–101.
12. Svien HJ, Mabon RF, Kernohan JW, Craig W. Ependymoma of the brain: pathologic aspects. Neurology. 1953. 3:1–15.
13. Tortori-Donati P, Fondelli MP, Cama A, Garre ML, Rossi A, Andreussi L. Ependymomas of the posterior cranial fossa: CT and MRI findings. Neuroradiology. 1995. 37(3):238–243.
14. Lee BCP, Kneeland JB, Cahill PT, Deck MDF. MR recognition of supratentorial tumors. AJNR Am J Neuroradiol. 1985. 6:871–878.
15. Komiyama M, Yagura H, Baba M, et al. MR imaging: possibility of tissue characterization of brain tumors using T1 and T2 values. AJNR Am J Neuroradiol. 1987. 8:65–70.
16. Coulon RA, Till K. Intracranial ependymomas in children: a review of 43 cases. Child's Brain. 1977. 3:154–168.
17. Furie DM, Provenzale JM. Supratentorial ependymomas and subependymomas: CT and MR appearance. J Comput Assist Tomogr. 1995. 19:518–526.
18. Li MH, Holtas S. MR imaging of spinal intramedullary tumors. Acta Radiol. 1991. 32:505–513.
19. Goy AMC, Pinto RS, Raghavendra BN, Epstein FJ, Kricheff II. Intramedullary spinal cord tumors: MR imaging, with emphasis on associated cysts. Radiology. 1986. 161:381–386.
20. Epstein FJ, Farmer JP, Freed D. Adult intramedullary spinal cord ependymomas: the result of surgery in 38 patients. J Neurosurg. 1993. 79:204–209.
21. Naidich TP, Zimmerman RA. Primary brain tumors in children. Semin Roentgenol. 1984. 19:100–114.
22. Nemoto Y, Inoue Y, Tashiro T, et al. Intramedullary spinal cord tumors: significance of associated hemorrhage at MR imaging. Radiology. 1992. 182:793–796.
23. Parizel PM, Bakeriaux D, Rodesch G, et al. Gd-DTPA-enhanced MR imaging of spinal tumors. AJR Am J Roentgenol. 1989. 152:1087–1096.
24. Johnson PC, Hunt SJ, Drayer BP. Human cerebral gliomas: correlation of postmortem MR imaging and neuropathologic findings. Radiology. 1989. 170:211–217.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download