Abstract
Objective
To compare sonography and mammography in terms of their diagnostic value in breast cancer cases which initially presented as an axillary mass without a palpable mass or other clinical symptoms.
Materials and Methods
Seven patients with enlarged axillary lymph nodes who first presented with no evidence of palpable breast lesions and who underwent both mammography and sonography were enrolled in this study. In six of the seven, the presence of metastatic adenocarcinoma was confirmed preoperatively by axillary needle aspiration biopsy; in four, subsequent sonographically-guided breast core biopsy performed after careful examination of the primary site indicated that primary breast cancer was present. In each case, the radiologic findings were evaluated by both breast sonography and mammography.
Results
Breast lesions were detected mammographically in four of seven cases (57%); in three of the four, the lesion presented as a mass, and in one as microcalcification. In three of these four detected cases, fatty or scattered fibroglandular breast parenchyma was present; in one, the parenchyma was dense. In the three cases in which lesions were not detected, mammography revealed the presence of heterogeneously dense parenchyma. Breast sonography showed that lesions were present in six of seven cases (86%); in the remaining patient, malignant microcalcification was detected at mammography. Final pathologic examination indicated that all breast lesions except one, which was a ductal carcinoma in situ, with microinvasion, were infiltrating ductal carcinomas whose size ranged from microscopic to greater than 3 cm. At the time of this study, all seven patients were alive and well, having been disease free for up to 61 months after surgery.
Axillary lymph node metastases without clinical or diagnostic evidence of a primary site have been seen as a manifestation of ipsilateral breast cancer, defined as 'occult'. Since Halstead reported three cases of occult breast cancer presenting as an axillary mass (1), the overall incidence of the condition has ranged from 0.3 to 0.8% (2). While the treatment for occult breast cancer is controversial, most studies have recommended mastectomy (3).
Efforts to detect occult breast lesions have frequently relied on the use of mammography. The results have been disappointing, however: primary tumors were discovered in more than 40% of patients whose mammograms were negative (4). Several recent reports have shown that otherwise occult primary breast cancers can be detected by MR imaging (5). In addition, however, the modality can assist in the selection of patients who are most likely to benefit from breast-conservation therapy, including initial chemoradiation. MR imaging is, however, inherently expensive, and, in any case, an MR image-guided localization system is not commercially available. For proper identification and biopsy of a lesion detected by MR imaging, directed post-MRI sonographic examination of the likely location of such a lesion must, therefore, be performed (6).
Few studies have compared the usefulness of fundamental modalities such as breast sonography and mammography for detecting and diagnosing occult breast lesions accompanied by axillary lymph node metastasis. In this study, breast cancers initially presenting as an axillary mass without other clinical symptoms were reviewed according to the radiologic findings, and breast sonography and mammography were compared in terms of their usefulness.
Between 1996 and 2000, the presence of breast cancer was pathologically proven in 1,445 patients who underwent biopsy or curative surgery at this institute. Using a radiologic database, ten patients (0.7%) were found to have enlarged axillary lymph nodes at initial clinical presentation, without evidence of palpable breast lesions, and in seven of these, who had undergone both mammography and sonography, the findings were jointly analyzed by two radiologists. The remaining three were excluded because their imaging studies were unavailable at the time of this investigation. All patients were female and aged between 43 and 64 (mean, 53.4) years.
Preoperatively, six of the seven patients involved underwent sonographically-guided needle aspiration biopsy of the axillary lymph nodes, and the presence of metastatic adenocarcinoma was demonstrated. The patient in whom biopsy was not performed underwent curative surgery. After the primary site was carefully determined by means of mammography and breast sonography, the breast nodules discovered were also biopsied. Pathological examination of all biopsies but one revealed the presence of infiltrating ductal cancer. All seven patients underwent curative resection of the primary lesions and three also received conjoined neoadjuvant chemotherapy. Surgery involved modified radical mastectomy in four patients, and partial mastectomy and axillary dissection in three.
The sonographic and mammographic findings were evaluated in each case, and clinical findings such as the pathology and size of a breast lesion, the duration and size of an axillary mass, and period of survival, were also determined. No patient involved in this study underwent MR imaging for the detection of occult breast lesions.
After initial clinical evaluation, palpable axillary masses persisted for approximately 10 days to 6 months. At both mammography and breast sonography, all clinically manifested axillary lesions were clearly visualized. They were either single (n=1) or conglomerated (n=6), measuring 1-3 cm in three patients and more than 3 cm in the other four.
As mentioned above, six of seven cases were confirmed at needle aspiration biopsy as metastatic adenocarcinoma. At subsequent sonographically-guided core biopsy of five patients with symptoms other than microcalcification, infiltrating ductal carcinoma was confirmed in four and benign fibrosis in one; in that patient the presence of infiltrating ductal carcinoma was proven by later curative surgery. Even though this had not been demonstrated at mammography, it was clearly visualized at sonography, measuring about 1.5×1.0 cm. Final pathologic examination of all seven ipsilateral cancer patients who underwent surgery showed that other than in one with a ductal carcinoma in situ, with microinvasion, all lesions were infiltrating ductal carcinomas.
Three of the seven primary tumors identified were less than 1 (mean, 0.6) cm in size, two measured 1-2 (mean, 1.4) cm, and two were larger than 2 (mean, 3.8) cm.
After surgery, all patients were followed up by means of periodic clinical and radiologic examinations. At the time of this study, all seven were alive and well, showing a good prognosis after surgery and with a postoperative disease-free survival time of up to 61 months.
The mammographic and sonographic findings are summarized in Table 1. The mammograms optained were suspicious in four cases (57%) [BIRADS category 4 (n=2) and 5 (n=2)] and negative in three. The suspicious mammograms demonstrated a mass in three patients (Fig. 1A) and microcalcification in one (Fig. 2A); fatty or scattered fibroglandular breast parenchyma was present in three, and dense parenchyma in one. In the three non-suspicious cases, mammography demonstrated heterogenously dense breast parenchyma (Fig. 3A).
At sonography, enlarged axillary lymph nodes were clearly visualized (Fig. 1B), and breast lesions in the form of a malignant nodule were observed in six (86%) of the seven cases (Figs. 1C, 3B). However, one patient in whom mammography revealed a malignant microcalcification showed a false negative finding at sonography.
In the absence of an obvious inflammatory lesion, persistent discrete axillary adenopathy among adult females is frequently the first sign of breast cancer. Fieuerman et al. (7) reported 14 cases of metastatic adenocarcinoma in an axillary lymph node, ten of which had metastasized from the breast. The sites of primary origin of axillary node metastasis, other than breast lesions, are known to include melanomas and carcinomas of the lung, thyroid, gastrointestinal tract, ovary, or other organs.
Breast cancer, while still in the intraductal stage and clinically obscure, appears to be able to metastasize to regional lymph nodes because of its ability to penetrate basement membrane (14). Indeed, a small primary breast tumor and a relatively larger metastatic mass exhibit differential growth potential, as shown in our cases.
It has been recommended that a carcinoma found in an axillary node should be treated as breast cancer, even if this is not clinically obvious (1, 8). In previously reported cases, the prognosis after radical mastectomy was better than if palpable breast cancer and axillary metastases remained (4), and at biopsy of a suspicious axillary node, a radiologist or clinician must therefore bear in mind the likelihood of breast cancer.
It is generally agreed that mammography is efficacious in detecting an 'occult' primary tumor. However, the reported frequency of detection of such tumors in this way has been disappointingly low (6). Positive mammographic findings appear to be of value in the detection of primary breast tumors, but where these findings are negative, the breast as the source of a primary tumor cannot be excluded.
Recently, with the development of MR technology, it has been demonstrated that the sensitivity of MR imaging in the detection of breast cancer is extremely high, ranging from 86% to 100% (9). In the future, because it can be of help in breast-sparing approaches to treatment, the use of MR imaging may more often be considered valuable. There are, however, there are several pitfalls. First, its specificity, at 37-86%, is lower and more variable than that of other modalities (10), and a MR imaging-guided localization system is not commercially available. In addition, the cost of breast MR imaging is high. Morris et al. reported a case in which a lesion was successfully identified and localized first by MR imaging and then by sonography (11).
It has been accepted that the primary role of sonography is the evaluation of palpable or mammographically identified masses. Owing to advances in sonograpahy-related technology, however, many investigators have reported cancers that were initially detected only at sonography. Kolb et al. (12), for example, demonstrated that screening sonography could depict small, early-stage, otherwise occult cancers in women with dense breasts, and that these lesions were not significantly different in size and stage from mammographically detected nonpalpable cancers. Similarly, Buchberger et al. (13) showed that the use of high-resolution sonography as an adjunct to mammograpahy in women with dense breasts may lead to the detection of a significant number of otherwise occult malignancies.
We found that the sensitivity of breast sonography in the detection of occult breast lesions, including three mammographically false-negative cases, was relatively high, though much time and effort were required. The only case that was not detected by sonography involved malignant microcalcification, though this was clearly demonstrated at mammography. Thus, mammography can be of assistance in detecting microcalcified lesions that are not clearly visible at sonography. In this study, mammography was found to have low efficacy in a dense breast.
Overall, breast sonography can play an important role in the evaluation of patients with axillary lymph node metastasis whose mammograms are negative. Breast sonography was shown to be superior to mammography in the detection and diagnosis of occult breast lesions, especially in a dense breast. Furthermore, the identification and localization of a lesion, and of a site for biopsy, can be better accomplished by breast sonography.
Figures and Tables
Fig. 1
A 64-year-old woman with axillary node metastasis.
A. Mammography reveals not only enlarged nodes but also scattered fibroglandular parenchyma, with a 1-cm spiculated nodule in the right upper outer quadrant (arrow).
B. At sonography, multiple enlarged axillary lymph nodes are seen, the largest of which has a diameter of approximately 3.5 cm.
C. At the corresponding site of mammographic abnormality, sonography depicts a 1cm-sized hypoechoic nodule, confirmed to be an infiltrating ductal carcinoma.
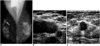
Fig. 2
A 48-year-old-woman with axillary node metastasis.
A. Mammography reveals the presence of clustered microcalcifications (arrows), without mass, in the right inner central area. At sonography, however, neither a breast nodule nor the above-mentioned microcalcifications are seen. Surgical excision after needle localization confirmed the presence of ductal carcinoma in situ, with microinvasion.
B. Axillary view depicts multiple hyperdense enlarged lymph nodes.
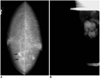
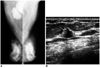
Fig. 3
A 60-year-old woman with axillary node metastasis.
A. Mammography demonstrates heterogeneous fibroglandular densities, with no sign of malignancy. The right axillary lymph nodes are, however, enlarged.
B. Careful examination of this sonogram shows that the right upper central area contains a malignant microlobulated nodule, 1cm in diameter (arrow). Sonograpahically-guided core biopsy confirmed the presence of an infiltrating ductal carcinoma.
References
1. Halsted WS. The results of radical operations for the cure of carcinoma of the breast. Ann Surg. 1907. 46:1–19.
2. Fourquet A, De La Rochefordiere A, Campana F. Harris JR, Lippman ME, Morrow M, Hellman S, editors. Occult primary cancer with axillary metastases. Diseases of the breast. 1996. Philadelphia: Lippincott-Raven;892–896.
3. Solin LJ. Fowble B, Goodman RL, Glick JH, Rosato EF, editors. Special considerations. Breast cancer treatment: a comprehensive guide to mangement. 1991. St Louis: Mosby-Year Book;523–528.
4. Patel J, Nemoto T, Rosner D, Dao TL, Pickren JW. Axillary lymph node metastasis from an occult breast cancer. Cancer. 1981. 47:2923–2927.
5. Orel SG, Schnall MD, Powell CM, et al. Staging of suspected breast cancer: effect of MR imaging and MR-guided biopsy. Radiology. 1995. 196:115–122.
6. Orel SG, Weinstein SP, Schnall MD, et al. Breast MR imaging in patients with axillary node metastases and unknown primary malignancy. Radiology. 1999. 212:543–549.
7. Fieuerman L, Attie JN, Rosenberg B. Carcinoma in axillary lymph nodes as an indicator of breast cancer. Surg Gynecol Obstet. 1962. 114:5–8.
8. Baron PL, Moore MP, Kinne DW, Candela FC, Osborne MP, Petrek JA. Occult breast cancer presenting with axillary metastases. Arch Surg. 1990. 125:210–215.
9. Orel SG, Hochman MG, Schnall MD, Reynolds C, Sullivan DC. High-resolution MR imaging of the breast: clinical context. RadioGraphics. 1996. 16:1385–1401.
10. Obdeijn IM, Brouwers-Kuyper EM, Tilanus-Linthorst MM, Wiggers T, Oudkerk M. MR imaging-guided sonography followed by fine-needle aspiration cytology in occult carcinoma of the breast. AJR. 2000. 174:1079–1084.
11. Morris EA, Schwartz LH, Dershaw DD, Van Zee KJ, Abramson AF, Liberman L. MR imaging of the breast in patients with occult primary breast carcinoma. Radiology. 1997. 205:437–440.
12. Kolb TM, Lichy J, Newhouse JH. Occult cancer in women with dense breasts: detection with screening US-Diagnostic yield and tumor characteristics. Radiology. 1998. 207:191–199.
13. Buchberger W, DeKoekkoek-Doll P, Springer P, Obrist P, Dunser M. Incidental findings on sonography of the breast: clinical significance and diagnostic workup. AJR. 1999. 173:921–927.
14. Ashikari R, Rosen PP, Urban JA, Senoo T. Breast cancer presenting as an axillary mass. Ann Surg. 1976. 183:415–417.
15. Stavros M, Buckley DL, Horsman A. Prediction of axillary lymph node status in invasive breast cancer with dynamic contrast-enhanced MR imaging. Radiology. 1997. 203:317–321.
16. Van Lancker M, Goor C, Sacre R, et al. Patterns of axillary lymph node metastasis in breast cancer. Am J Clin Oncol. 1995. 18:267–272.
17. Kambouris AA. Axillary node metastasis in relation to the size and location of breast cancers: analysis of 147 patients. Am Surg. 1996. 62:519–524.
18. Rosen EL, Sickles E, Keating D. Ability of mammography to reveal nonpalpable breast cancer in women with palpable breast masses. AJR. 1999. 172:309–312.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download