Abstract
Nontuberculous mycobacterial (NTM) infections are an increasingly recognized cause of chronic lung disease in immunocompetent adults, and the M. avium complex, M. kansasii, and a rapidly growing mycobacteria such as M. abscessus, M. fortuitum, and M. chelonae account for most of the pathogens involved. Because the clinical features of NTM disease are not distinguishable from those of tuberculosis, and NTM are ubiquitous in the environment, diagnosis requires that the bacilli are isolated and identified. NTM diseases have been difficult to treat, though since the introduction of new macrolides, the outcome for patients with some NTM diseases has improved significantly. For correct diagnosis and the successful treatment of NTM pulmonary disease, a knowledge of the full spectrum of clinical and radiological findings is important.
The clinical importance of tuberculosis distinguishes the Mycobacterium tuberculosis (M. tuberculosis) complex from all other mycobacteria. Except for the M. tuberculosis complex and M. leprae, mycobacteria are referred to collectively as nontuberculous mycobacteria (NTM); previous names for this group of organisms include 'environmental mycobacteria,' 'atypical mycobacteria,' or 'mycobacteria other than tuberculosis,' though the term NTM is preferred (1, 2).
Unlike M. tuberculosis, which is an obligate human pathogen with no environmental reservoir, NTM are commonly isolated from environmental sources such as water and soil, and until the second half of the previous century, occasional isolates of NTM were thus largely considered contaminants or colonizers (1, 3). Some NTM, however, are pathogens that may cause severe disease or even death (1-3).
Human disease due to NTM is classified into four distinct clinical syndromes: pulmonary disease, lymphadenitis, cutaneous disease, and disseminated disease; among these, chronic pulmonary disease is the most common localized clinical condition (1, 2). The radiologic manifestations of NTM pulmonary disease vary and are often subtle, and may be indistinguishable from those of tuberculosis. For correct diagnosis and the successful treatment of the various conditions which may present, a knowledge of the full spectrum of clinical and radiologic findings is important.
In this article, the authors review the microbiology and epidemiology of NTM species, and the clinical and radiological manifestations, diagnosis and treatment of the pulmonary diseases they give rise to, especially in human immunodeficiency virus (HIV)-negative patients.
Within the genus Mycobacterium, four groups of human pathogens can be delineated on the basis of their microbiologic, clinical, and epidemiologic characteristics (Table 1). The traditional Runyon classification of NTM recongnizes these four groups on the basis of growth rates, colony morphology, and pigmentation (the Runyon Classification System). Groups I, II and III are considered slow growers, requiring a time similar to that required by M. tuberculosis to grow in culture, while Group IV organisms are rapid growers which grow well in routine bacteriologic media in less than seven days. The slow growers are further differentiated according to their ability to produce yellow pigment (1).
This classification system has been primarily a tool for microbiologists, and has allowed easier identification of individual NTM species by mycobacterial laboratories. However, the Runyon classification has become less relevant in recent years due to advances in mycobacteriology, including more rapid culturing techniques, DNA probes, and high-pressure liquid chromatography. In addition, this system is of little value to the clinician, because the organisms in a particular Runyon class may cause quite different patterns of clinical disease. A more appropriate grouping for these organisms is currently based on the type of clinical disease they produce: pulmonary disease, lymphadenitis, cutaneous disease, and disseminated disease (1, 2).
Most NTM are generally environmentally ubiquitous and have been recovered from water and soil; in humans they are low-grade pathogens. Person-to-person transmission of infection is rare, and isolation of infected individuals is thus not required. It is generally accepted that most human infection is due to environmental NTM, and that airborne NTM infection may play an important role in respiratory disease (1, 2).
There is marked geographic variability both in the prevalence of disease and in the mycobacterial species responsible for it. Pulmonary NTM disease in the United States is most commonly due to the M. avium complex (MAC), with M. kansasii in second place (1). In the United Kingdom, M. kansasii is the most common pathogen in NTM lung disease in England and Wales, while M. malmoense is the most common in Scotland. In south-east England, M. xenopi predominates (2). In Japan, the most common cause of NTM pulmonary disease is MAC, followed by M. kansasii (4).
It is very interesting that the epidemiologic data reported from Japan are very similar to those from the United States. Between 1983 and 1984 in Japan, MAC was identified in approximately 70% of patients with NTM, while M. kansasii was identified in roughly 25% of such cases (4). Between 1981 and 1983 in the United States, 62% of NTM cases were associated with MAC and 24% with M. kansasii (5). The incidence rates are also very similar between the two countries: the reported incidence of MAC lung disease per 100,000 population per year is 1.29 in Japan and 1.28 in U.S.A., while for M. kansasii, the corresponding figures are 0.34 and 0.33 (4).
In Korea, the reported number of NTM cases has been rising since the 1980s, though precise epidemiologic data is not yet available. Earlier investigations of the distribution of environmental mycobacteria demonstrated that a variety of NTM species including MAC, M. fortuitum, and M. chelonae were isolated from Korean soil and water samples (6). In another report investigating NTM infection in schoolchildren, involving skin testing of an NTM antigen comprising M. intracellulare, M. fortuitum, and M. scrofulaceum, significant skin reaction was observed in approximately 10 to 15% of subjects (7).
The first case report of MAC pulmonary disease in Korea was published in 1981 (8), and a number of cases of pulmonary disease caused by MAC (9), M. abscessus (10, 11), M. chelonae (12), M. fortuitum (9) and M. celatum (13) in HIV-seronegative adults have since been reported. Using microbiological and clinical data, several papers have detailed the relative frequency of isolated NTM (Tables 2, 3) (14-17). Synthesis of these various reports shows that the pathogen most frequently responsible for NTM pulmonary disease in Korea is MAC, followed by members of the M. fortuitum and M. chelonae group (including M. abscessus, formerly known as M. chelonae, subspecies abscessus) (14, 15, 17).
Interestingly, the only case report of M. kansasii pulmonary disease in Korea involved disseminated M. kansasii infection in a patient with idiopathic CD4+ T-lymphocytopenia (18). In Japan, as previously mentioned, pulmonary disease caused by M. kansasii has been increasing since the last 1970s, becoming the second most common type of NTM pulmonary disease after MAC (4, 19).
Unlike M. tuberculosis, NTM are not obligate pathogens. Accordingly, the isolation of an NTM species from a respiratory sample is not sufficient evidence of the presence of NTM lung disease, the diagnosis of which is based on clinical, radiographic, and bacteriologic criteria (1).
The necessary clinical criterion is the presence of compatible symptoms and signs, with the reasonable exclusion of other etiologies of pulmonary disease. However, the signs and symptoms of NTM lung disease are often variable and nonspecific. Patients fraquently present with chronic cough, productive sputum, and fatigue. NTM infection of the lungs often occurs in the context of preexisting lung disease, especially chronic obstructive pulmonary disease, bronchiectasis, pneumoconiosis, and previous tuberculosis. As a result, the clinical manifestations of NTM lung disease are often similar to those of the underlying disease (1).
The radiographic criteria required are the presence of infiltrates, cavitation, or multiple nodules at plain chest radiography, and/or multiple small nodules less than 10 mm in diameter or multifocal bronchiectasis at high-resolution computed tomography (HRCT) of the lungs (Figs. 1, 2, 3, 4, 5, 6), findings which depend in part on the species involved in pulmonary infection (Table 4). The well-known radiographic features of NTM lung disease caused by MAC and M. kansasii are similar to those of postprimary tuberculosis (20-23). In these classic upper lung zone diseases, the most common findings are linear and nodular areas of increased opacity in the apical and posterior segments of the upper lobes. In upper lung zone disease, cavitation occurs in about 95% of patients with M. kansasii infection and approximately 75% of those with MAC infection, frequencies similar to those found in pulmonary tuberculosis (21, 22). NTM tend to give rise to thinner-walled cavities and less surrounding parenchymal infiltration than are found in tuberculosis cases (20, 24), though these classic forms of MAC or M. kansasii lung disease may be indistinguishable from pulmonary tuberculosis (20, 22, 23).
Recent studies involving HRCT of the chest have shown that in many patients with non-cavitary disease of the middle and lower lung zone caused by MAC pulmonary infection, both multifocal bronchiectasis and clusters of small nodules and branching linear structures are present (25-28) (Figs. 2, 4, 6).
In the absence of diagnostic specificity of the clinical manifestations or chest radiographic findings, the diagnosis of NTM pulmonary disease requires microbiologic confirmation. Positive sputum cultures for NTM must, however, be interpreted cautiously. The discovery of NTM in a single sputum sample is not proof of NTM disease, especially when the acid-fast-bacillus smear is negative and NTM are cultured in small numbers. The distinction between colonization or contamination and true infection is often difficult and somewhat arbitrary.
In 1997, the American Thoracic Society issued a revised statement of diagnostic criteria for NTM lung disease (1) (Table 5), and in 2000, the British Thoracic Society published guidelines for the management of NTM disease (2). According to the British guidelines, which had less strict diagnostic criteria than those of the American Thoracic Society statement, NTM pulmonary disease is diagnosed when positive cultures develop from specimens of sputum obtained at least seven days apart (positive culture, twice) from a patient whose chest radiograph suggests mycobacterial infection and who may or may not present with symptoms or signs (2).
The American Thoracic Society diagnostic criteria put relatively great emphasis on multiple culture and identification using at least three sputum samples; the invasive bronchoscopic approach includes bronchial washing and transbronchial lung biopsy and HRCT, especially in patients without cavitary infiltrates. These criteria were considered by some investigators as being primarily designed for use in the United States, where the incidence of tuberculosis is low and the relative incidence of NTM pulmonary disease is high (29). In developing countries, where the incidence of pulmonary tuberculosis is much higher than that of NTM pulmonary disease, the initiation of presumptive antituberculous treatment, especially in smear-positive patients prior to the identification of isolates, is common practice. With empirical first-line antituberculous treatment, early sputum conversion to culture-negativity would be expected in some cases of NTM pulmonary disease, reducing the likely success of attempts to obtain further positive culture of isolates. Thus, patients in whom the isolate of NTM has been initially identified but who show less than three isolations from their sputum examinations cannot meet the American Thoracic Society's diagnostic criteria. In developing countries, where mycobacterial culture and identification of multiple specimens of sputum are not routine practice because of the high incidence of tuberculosis and high cost of multiple mycobacterial culture and identification, a solution to these problems would be of particular value to clinicians (30). Whether the American Thoracic Society's diagnostic approach could be feasible for other developing countries such as Korea is not yet clear, and remains to be demonstrated by the findings of further studies.
In general, evidence of disease, such as infiltration visible at chest radiography, the cause of which has not been determined by careful clinical and laboratory studies, and the repeated isolation of multiple colonies of the same strain of NTM in the absence of other pathogens, are sufficient for the diagnosis of NTM pulmonary disease (1).
Because NTM pulmonary disease can be quite indolent, the importance of appropriate follow-up to determine the significance of potentially pathogenic NTM isolated from sputum cannot be overemphasized. Delays in diagnosis are frequent, and radiographs may remain unchanged for years: in one series, an average of 6.4 years passed before radiographic change was apparent (31). Where NTM cultures are positive, stable findings at chest radiography, especially at relatively short intervals, are not sufficient grounds to exclude infection. In the absence of lung biopsy, months to years of clinical, radiographic, and microbiological follow-up of certain patients may be required to reliably determine the significance of NTM respiratory isolates (1).
The methods of acid-fast staining and culture currently used to discover M. tuberculosis are acceptable for most NTM species. The appearance of NTM at microscopy is generally indistinguishable from that of M. tuberculosis, and the American Thoracic Society has recommended that samples should be inoculated onto at least one solid medium (Lowenstein-Jensen or Middlebrook 7 H10 and 7 H11) and into a liquid culture system (BACTEC, MGIT, ESP); the latter allows more rapid culture and isolation of a greater range of species than does the use of solid media alone (1), though at present, many microbiology laboratories in Korea use only Lowenstein-Jensen solid media.
NTM are identified by their pattern of pigmentation, growth characteristics, microscopic appearance, and biochemical reactions. More rapid discriminating systems are being developed, and include DNA probes, high-performance liquid chromatography, polymerase chain reaction restriction enzyme analysis, and 16S rRNA gene sequence analysis (1, 2).
In NTM infections, susceptibility testing is more difficult and more controversial than in those due to M. tuberculosis. In general, the results of standard susceptibility tests are of little or no value in predicting clinical efficacy in NTM infections, and the provision of in-vitro susceptibility results to clinicians is likely to be confusing rather than helpful (1, 2). Both the American and the British Thoracic Society have recommended that routine testing of the susceptibility of NTM to antituberculous drugs be discouraged (1, 2).
MAC is the most commonly isolated and most clinically important pulmonary NTM pathogen, and includes the two species M. avium and M. intracellulare. The fact that they are distinct has no clinical or prognostic value for individual patients, however, and they are generally not differentiated.
The symptoms and signs of MAC lung disease are variable and nonspecific and, in addition, the natural history of MAC lung disease in HIV-negative patients is unpredictable. Some show a stable clinical and radiographic picture for years, while in others, progression of their disease is relatively rapid. This feature appears to relate in part to the existence of two types of clinical disease and presentation.
MAC pulmonary disease has been recently differentiated into two distinct subtypes, the upper lobe cavitary form (Fig. 1) and the nodular bronchiectatic form (Fig. 2) (1). The former, the traditional and most widely known presentation of MAC pulmonary disease, is usually seen in white, middle-aged or elderly men who smoke or abuse alcohol. Underlying disorders commonly include chronic obstructive pulmonary disease, previous tuberculosis, and silicosis. Chest radiography frequently demonstrates apical cavitary change similar to that seen in reactivated tuberculosis (20-23, 32). Cavitation is common and frequently associated with apical pleural thickening; the cavities are usually small and thin-walled (20, 27, 28). Endobronchial spread of disease is also common and manifests as unilateral or bilateral scattered nodular areas of increased opacity (Fig. 1). Adenopathy and pleural effusion are uncommon, though progressive fibrosis with volume loss and traction bronchiectasis in the upper lobes occurs in one-third of patients (22, 23, 32). This form of disease is generally progressive, and if left untreated can lead to extensive lung destruction and death (1).
A second and less dramatic clinical presentation, the so-called nodular bronchiectatic form, has recently been recognized (1) (Fig. 2), and occurs predominantly in nonsmoking middle-aged or elderly women who also present with chronic cough and sputum production. Interestingly, previous or underlying lung disease has not been noted in these patients (33, 34). In addition, the radiographic findings are quite distinct from those of the classical upper lobe cavitary form of the disease: the characteristic findings are bilateral nodular or interstitial/nodular change, predominantly in the lower lung zones, and particularly in the right middle lobe and lingular segment of the left upper lobe (33, 34). The apical pattern resembling reactivated tuberculosis is not present.
This type of disease often used to be referred to as airway colonization, where the real underlying condition was bronchiectasis. Several previous investigators have supported the concept of "colonization" (defined as isolation of the organism from the respiratory tract, without evidence of tissue invasion) (21), and recent studies involving the use of HRCT scanning have indicated that these patients show specific radiographic features of parenchymal disease in addition to multifocal areas of bronchiectasis. Typical HRCT findings are multiple small nodules (< 5 mm) and branching linear structures combined with bronchiectasis in the same lobe of the lung, features which are usually confined to or most severe in the right middle lobe and lingular segment of the left upper lobe (Fig. 2) (25-28). In addition, transbronchial lung biopsy specimens show granulomatous inflammation, suggesting lung tissue invasion by the organisms involved (35). One recent study demonstrated that the characteristic pathologic finding of pulmonary MAC disease is extensive granuloma formation throughout the airways (36). Both the HRCT and the pathologic findings are considered to be due to the presence of mycobacterial disease, and the term 'colonization' may, therefore, be inappropriate (1).
In nodular bronchiectasis, isolation of MAC from sputum specimens is less consistent than in the upper lobe cavitary form of the disease: sputum may be intermittently positive and/or positive, with low numbers of organisms. A recently published report showed that because of high false-negative rates of sputum cultures in such a population, 45% of patients required bronchoscopy or lung biopsy to diagnose active MAC infection (37). This low sensitivity of sputum cultures may result from the non-cavitary nature of the disease.
In some patients, the condition initially involves the presence of small peripheral nodules, with the subsequent development of infiltrate and bronchiectasis in adjacent parenchyma (35). One study using serial CT examinations demonstrated the progression of existing bronchiectasis as well as the formation of new areas (38), results which strongly suggest that in some patients at least, bronchiectasis may not only be a predisposing condition for MAC infection but also be caused by it.
The nodular bronchiectatic form of MAC disease tends to progress much more slowly than the cavitary form, and over time may gradually lead to respiratory failure. In the original report of this disease (33), the condition was progressive in eight (38%) of the 21 immunocompetent adult patients involved. Four (50%) of the eight died after progression during the longitudinal follow-up period led to respiratory failure (33). Patients with this new clinical presentation of MAC pulmonary disease accounted for 25% of the total number of HIV-negative cases in that first report, and subsequent reviews have indicated that this patient population accounts for 50% of MAC pulmonary disease cases (39).
Historically, medical treatment of MAC pulmonary disease in HIV-negative patients has been disappointing. Before the introduction the macrolides in the 1990s, multi-drug regimens, usually including isoniazid, rifampin, ethambutol and streptomycin, had been recommended (40): this combination therapy provided initial sputum conversion rates of approximately 50 to 70%, with the long-term success rate of less than 50% mainly due to treatment failure and relapses (1, 41). The newer macrolides, clarithromycin and azithromycin, have had a great impact on the treatment of this disease, with recent studies showing excellent in-vitro and clinical results (42-44). For the treatment of adults without HIV infection, the American Thoracic Society recommended a regimen of clarithromycin or azithromycin, rifampin or rifabutin, and ethambutol, to be taken daily. Streptomycin should also be considered, especially for patients who have radiographically extensive or cavitary disease, and particularly when this is accompanied by strongly positive sputum smears (1).
Reports of experiences with these regimens have mentioned sputum conversion rates of up to 90% in patients with no history of previous treatment failure and tolerance of all three oral drugs (42-44). All patients treated with macrolides show clinical improvement within 3 to 6 months, and sputum conversion occurs within 12 months (43). Treatment is continued until sputum cultures are consecutively negative for at least one year (1).
Unlike other NTM, M. kansasii has never been found in soil or natural water supplies, but has been discovered in piped water systems in cities where it is endemic. Previous studies have demonstrated that M. kansasii disease is concentrated in urban areas, supporting a possible association between clinical disease and the presence of the organism in potable water supplies (1).
The clinical and radiological features of pulmonary disease caused by M. kansasii usually resemble those of pulmonary tuberculosis. Such disease presents more frequently in older men, and a history of cigarette abuse and chronic obstructive pulmonary disease is found in more than 50% of patients (45).
The radiographic features of M. kansasii pulmonary disease are very similar to those of pulmonary tuberculosis. Cavitation occurs in about 90% of cases, with a preponderance of upper lobe involvement (Fig. 3), and in some cases, the cavities tend to have thinner walls and less surrounding parenchymal infiltration than in tuberculosis (21, 46). The differences are not sufficient to permit differential diagnosis on the basis of the radiographic findings alone, though the presence of pleural effusion or lower lobe involvement makes M. kansasii infection very unlikely (47).
The natural history of pulmonary disease caused by M. kansasii in patients receiving no drug treatment has shown persistent sputum production and progressive clinical and radiologic disease (1). With rifampin-containing regimens, however, sputum conversion rates are almost 100%, and treatment failure and long-term relapse rates are very low (48). The American Thoracic Society recommendation for treatment of pulmonary disease caused by M. kansasii includes isoniazid, rifampin, and ethambutol. Medication is usually administered daily for 18 months, and a minimum of 12 months of negative sputum cultures indicates successful treatment (1).
Most clinical pulmonary disease is due to three clinically relevant species of rapidly growing mycobacteria (RGM): M. abscessus, M. fortuitum, and M. chelonae. Among the pulmonary diseases these cause, M. abscessus (formerly Mycobacterium chelonae, subspecies abscessus) is responsible for approximately 80% of isolates, and M. fortuitum for a further 15% (49, 50).
RGM pulmonary disease patients tend to be middle-aged or older, female, and nonsmokers (49). Specific underlying diseases, which are infrequent and occur in only approximately 20% of patients, include prior mycobacterial infection, gastroesophageal disorders with chronic vomiting, and bronchiectasis (49, 50). As in other forms of NTM lung disease, the symptoms are indolent and diagnosis is usually not established until more than two years after the thier onset (49).
The most frequent patterns seen at chest radiography are interstitial, interstitial/alveolar, and/or reticulonodular densities (Figs. 4-6). Cavitation occurs in only 15% of patients (49). The disease is typically multilobar and bilateral, with slight upper lobe predominance (49, 51). The most common CT findings in patients with M. chelonae infection are diffuse bronchiectasis, multiple small lung nodules, focal areas of consolidation, and bronchial wall thickening, and are similar to those reported for MAC pulmonary disease (52) (Fig. 6).
In most patients with M. abscessus without underlying disorder, the disease progresses very slowly, and some patients show little radiographic change over a period of years. According to one report, in which the outcomes of 154 patients with RGM pulmonary disease were reviewed, death was attributed directly to progressive RGM lung disease, accompanied by respiratory failure in 21 cases (14%) (49).
The treatment of M. abscessus is complex. Isolates are generally susceptible only to parenteral antibiotics such as amikacin, cefoxitin and imipenem, and the newer oral macrolides, though the organism is very difficult to eradicate. A report of 154 patients infected with RGM (49) stated that only ten infected with M. abscessus were cured. Seven of these ten received parenteral antibiotics for 1 to 3 months, followed by surgical excision, whereas only three were successfully treated with antibiotics alone. Surgical resection of localized disease is thought to be the most effective way of achieving complete eradication of the organism (1, 49).
M. fortuitum, which is usually more susceptible to multiple oral antimicrobial agents, including the newer macrolides and quinolones, doxycycline and minocycline, and sulfonamides, requires a different approach, and for this species, drug susceptibility testing is crucial. Clinical cure usually results from 6 to 12 months of therapy with two oral agents to which the M. fortuitum isolate is susceptible in vitro (1).
The incidence of NTM pulmonary diseases is increasing, while that of tuberculosis is decreasing. In Korea, the M. avium complex and rapidly growing mycobacteria such as M. abscessus, M. chelonae, and M. fortuitum are reported to account for most of the pathogens responsible for NTM pulmonary infection. Because of the low sensitivity of sputum smears and culture, diagnosis of NTM pulmonary disease, especially in its non-cavitary forms, is often difficult. In patients in whom chest radiographs depict bilateral bronchiectasis and reticulonodular infiltrates that are confined to or most severe in the right middle lobe and lingular segment of the left upper lobe, NTM pulmonary disease, especially nodular bronchiectatic forms of MAC diseases, should be suspected, and an aggressive diagnostic approach including HRCT and bronchoscopy should be considered. For the correct diagnosis and treatment of NTM pulmonary disease, physicians and radiologists must fully understand the various clinical and radiographic findings described.
Figures and Tables
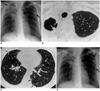 | Fig. 1A 46-year-old woman with M. avium infection.
A. Initial chest radiograph shows cavitary lesions (arrows) in both upper lobes. Also note that bilateral reticulonodular lesions are present in upper and middle lung zones.
B. High-resolution (1.0-mm collimation) CT scan obtained at the level of the thoracic inlet, and at the same time as A, depicts a cavitary lesion with pleural thickening in the right apex. Nodules (arrows) project from the wall of the cavitation into its lumen.
C. CT scan obtained at the level of the inferior pulmonary vein shows centrilobular nodules and branching linear structures (arrows) in both lungs, suggesting the bronchogenic spread of disease. Also note the presence of bronchiectasis in the right middle lobe.
D. Follow-up radiograph obtained 31 months after A, and after treatment, shows some increase in the extent of reticulonodular lesions, especially in left upper and middle lung zones (arrows).
|
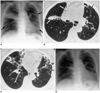 | Fig. 2A 67-year-old woman with M. avium infection.
A. Initial chest radiograph reveals airspace consolidation in the right upper lobe and reticulonodular lesions (arrows) in the right middle, and left middle and lower, lung zones.
B. High-resolution (1.0-mm collimation) CT scan obtained at the level of the bronchus intermedius, and at the same time as A, shows air-space consolidation (arrows) at the bottom of the right upper lobe, and bronchiectasis and small centrilobular nodules (small arrows) in the lingular segment of the left upper lobe.
C. CT scan obtained at the level of the right basal trunk depicts centrilobular nodules and branching linear structures (arrows), an acinar nodule (curved arrows), lobular consolidation (open arrows), and bronchiectasis.
D. Follow-up radiograph obtained 31 months after A, and after treatment, shows that in both lungs, disease is less extensive.
|
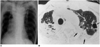 | Fig. 3A 78-year-old man with M. kansasii infection.
A. Chest radiograph reveals the presence of reticulonodular lesions in both upper lobes, which have decreased in volume. Also note right apical pleural thickening and emphysematous overinflation in remaining lung areas.
B. High-resolution (1.0-mm collimation) CT scan obtained at the level of the aortic arch shows consolidation containing the open bronchus sign in the right upper lobe and thin-walled cavity (small arrows), and some areas of consolidation (arrows) in the left upper lobe. Also note the reduced volume of both upper lobes.
|
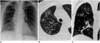 | Fig. 4A 54-year-old woman with M. abscessus infection.
A. Chest radiograph depicts patchy areas of reticulonodular lesions in the entire right and left upper lung.
B. High-resolution (1.0-mm collimation) CT scan obtained at the level of the aortic arch demonstrates bronchiectasis, small nodules, and ground-glass opacity in the right upper lobe.
C. Reformed coronal image depicts bronchiectasis, small nodules, and ground-glass opacity in the entire right lung.
|
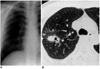 | Fig. 5A 33-year-old man with M. fortuitum infection.
A. Chest radiograph shows focal opacification of the right upper lung zone.
B. High-resolution (1.0-mm collimation) CT scan obtained at the level of the aortic arch shows nodules of various sizes in the right upper lobe. The dominant nodule contains internal cavitation (arrow).
|
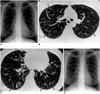 | Fig. 6A 37-year-old woman with M. chelonae infection.
A. Initial chest radiograph depicts reticulonodular lesions in both lungs, especially in the upper and middle zones.
B. High-resolution (1.5-mm collimation) CT scan obtained at the level of the main bronchi, and at the same time as A, shows centrilobular nodules and branching linear structures (arrows) in both lungs.
C. CT scan obtained at the level of the right inferior pulmonary vein reveals bronchiectasis in both lungs, which contain centrilobular nodules (arrows).
D. Follow-up radiograph obtained 58 months after A shows that as a result of treatment, disease is less extensive.
|
Table 2
Frequency of Occurrence of Nontuberculous Mycobacteria Isolated from Clinical Specimens Submitted to the Mycobacterial Laboratory of the Korean Institute of Tuberculosis

Table 3
Frequency of Occurrence of Isolates of Nontuberculous Mycobacteria in Patients with Proven Pulmonary Disease According to Published Case Series Studies in Korea

References
1. American Thoracic Society. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am J Respir Crit Care Med. 1997. 156:S1–S25.
2. British Thoracic Society. Management of opportunistic mycobacterial infections: Joint Tuberculosis Committee guidelines, 1999. Thorax. 2000. 55:210–218.
3. Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979. 119:107–159.
4. Tsukamura M, Kita N, Shimoide H, Arakawa H, Kuze A. Studies on the epidemiology of nontuberculous mycobacteriosis in Japan. Am Rev Respir Dis. 1988. 137:1280–1284.
5. O'Brien RJ, Geiter LJ, Snider DE Jr. The epidemiology of nontuberculous mycobacterial diseases in the United States: results from a notional survey. Am Rev Respir Dis. 1987. 135:1007–1014.
6. Jin BW, Saito H, Yoshii Z. Environmental mycobacteria in Korea: I. Distribution of the organisms. Microbiol Immunol. 1984. 28:667–677.
7. Ministry of Health & Social Affairs. Korean National Tuberculosis Association. The 2nd report on the 6th tuberculosis prevalence survey in Korea. 1990. Seoul: 1–11. (in Korean).
8. Kim SJ, Hong YP, Kim SC, Bai GH, Jin BW, Park CD. A case of pulmonary disease due to Mycobacterium avium-intracellulare complex. Tuberc Lung Dis. 1981. 28:121–124. (in Korean).
9. Kim SJ, Hong YP, Bai GH, Kim SC, Jin BW. Nontuberculous pulmonary infection in two patients with Mycobacterium avium-intracellulare complex and a patient with M. fortuitum. J Kor Soc Microbiol. 1982. 17:87–93. (in Korean).
10. Kim HJ, Oh SH, Lee WY, Kim SJ. Report of a case of pulmonary mycobacteriosis caused by Mycobacterium chelonei subsp. abscessus. Tuberc Lung Dis. 1985. 32:54–57. (in Korean).
11. Yim JJ, Oh MD, Yoo CG, et al. A case of Mycobacterium abscessus pneumonia in a patient with systemic lupus erythematosus. Tuber Lung Dis. 1999. 46:96–102. (in Korean).
12. Kim EK, Shim TS, Lim CM, et al. Clinical features of Mycobacterium chelonae pulmonary infection. Tuberc Lung Dis. 2001. 51:Suppl 2. 85. (abstract). (in Korean).
13. Kim DK, Kim BJ, Kook YH, et al. Pulmonary infection with Mycobacterium celatum in immunocompetent host: first case report in Korea. Tuberc Lung Dis. 1999. 47:697–703. (in Korean).
14. Bai GH, Park KS, Kim SJ. Clinically isolated mycobacteria other than Mycobacterium tuberculosis from 1980 to 1990 in Korea. J Korean Soc Microbiol. 1993. 28:1–5. (in Korean).
15. Lew WJ, Ahn DI, Yoon YJ, et al. Clinical experience of mycobacterial diseases other than tuberculosis. Tuberc Lung Dis. 1992. 39:425–432. (in Korean).
16. Korean Academy of Tuberculosis and Respiratory Diseases. National survey of mycobacterial diseases other than tuberculosis in Korea. Tuberc Lung Dis. 1995. 42:277–294. (in Korean).
17. Yoon CJ, Goo JM, Seo JB, Kim SH, Im JG. CT findings of mycobacterial infection other than tuberculosis: comparison with tuberculosis. J Korean Radiol Soc. 2000. 42:487–492. (in Korean).
18. Park SY, Park JH, Jegal YJ, et al. A case of idiopathic CD4+ T-lymphocytopenia with disseminated Mycobacterium kansasii infection and pulmonary alveolar proteinosis. Tuberc Respir Dis. 2000. 48:377–382. (in Korean).
19. The Mycobacteriosis Research Group of the Japanese National Chest Hospitals. Rapid increase of the incidence of lung disease due to Mycobacterium kansasii in Japan. Chest. 1983. 83:890–892.
20. Christensen EE, Dietz GW, Ahn CH, et al. Initial roentgenographic manifestations of pulmonary Mycobacterium tuberculosis, M. kansasii, and M. intracellularis infections. Chest. 1981. 80:132–136.
21. Ahn CH, McLarty JW, Ahn SS, Ahn SI, Hurst GA. Diagnostic criteria for pulmonary disease caused by Mycobacterium kansasii and Mycobacterium intracellulare. Am Rev Respir Dis. 1982. 125:388–391.
22. Miller WT Jr. Spectrum of pulmonary nontuberculous mycobacterial infection. Radiology. 1994. 191:343–350.
23. Erasmus JJ, McAdams HP, Farrell MA, Patz EF Jr. Pulmonary nontuberculous mycobacterial infection: radiologic manifestations. RadioGraphics. 1999. 19:1487–1505.
24. Woodring JH, Vandiviere HM. Pulmonary disease caused by nontuberculous mycobacteria. J Thorac Imaging. 1990. 5:64–76.
25. Hartman TE, Swensen SJ, Williams DE. Mycobacterium avium-intracellulare complex: evaluation with CT. Radiology. 1993. 187:23–26.
26. Swensen SJ, Hartman TE, Williams DE. Computed tomographic diagnosis of Mycobacterium avium-intracellulare complex in patients with bronchiectasis. Chest. 1994. 105:49–52.
27. Lynch DA, Simone PM, Fox MA, Bucher BL, Heinig MJ. CT features of pulmonary Mycobacterium avium complex infection. J Comput Assist Tomogr. 1995. 19:353–360.
28. Primack SL, Logan PM, Hartman TE, Lee KS, Müller NL. Pulmonary tuberculosis and Mycobacterium avium-intracellulare: a comparison of CT findings. Radiology. 1995. 194:413–417.
29. Corbett EL, Blumberg L, Churchyard GJ, et al. Nontuberculous mycobacteria: defining disease in a prospective cohort of South African miners. Am J Respir Crit Care Med. 1999. 160:15–21.
30. World Health Organization. Global Tuberculosis Programme. Publication reference WHO/GTB/96.210. Treatment of Tuberculosis: Guidelines for National Programmes. 1997. 2nd ed. Geneva, Switzerland: World Health Organization.
31. Woodring JH, Vandiviere HM, Melvin IG, Dillon ML. Roentgenographic features of pulmonary disease caused by atypical mycobacteria. South Med J. 1987. 80:1488–1497.
32. Christensen EE, Dietz GW, Ahn CH, et al. Pulmonary manifestations of Mycobacterium intracellulare. AJR. 1979. 133:59–66.
33. Prince DS, Peterson DD, Steiner RM, et al. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989. 321:863–868.
34. Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. Chest. 1992. 101:1605–1609.
35. Tanaka E, Amitani R, Niimi A, Suzuki K, Murayama T. Yield of computed tomography and bronchoscopy for the diagnosis of Mycobacterium avium complex pulmonary disease. Am J Respir Crit Care Med. 1997. 155:2041–2046.
36. Fujita J, Ohtsuki Y, Suemitsu I, et al. Pathological and radiological changes in resected lung specimens in Mycobacterium avium-intracellulare complex disease. Eur Respir J. 1999. 13:535–540.
37. Huang JH, Kao PN, Adi V, Ruoss SJ. Mycobacterium avium-intracellulare pulmonary infection in HIV-negative patients without preexisting lung disease: diagnostic and management limitations. Chest. 1999. 115:1033–1040.
38. Moore EH. Atypical mycobacterial infection in the lung: CT appearance. Radiology. 1993. 187:777–782.
39. Wallace RJ Jr, Zhang Y, Brown BA, et al. Polyclonal Mycobacterium avium complex infections in patients with nodular bronchiectasis. Am J Respir Crit Care Med. 1998. 158:1235–1244.
40. American Thoracic Society. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am Rev Respir Dis. 1990. 142:940–953.
41. BritishThoracic Society. First randomised trial of treatments for pulmonary disease caused by M. avium-intracellulare, M. malmoense, and M. xenopi in HIV negative patients: rifampicin, ethambutol and isoniazid versus rifampicin and ethambutol. Thorax. 2001. 56:167–172.
42. Wallace RJ Jr, Brown BA, Griffith DE, Girard WM, Murphy DT. Clarithromycin regimens for pulmonary Mycobacterium avium complex: The first 50 patients. Am J Respir Crit Care Med. 1996. 153:1766–1772.
43. Dautzenberg B, Piperno D, Diot P, Truffot-Pernot C, Chauvin JP. Clarithromycin in the treatment of Mycobacterium avium lung infections in patients without AIDS. Chest. 1995. 107:1035–1040.
44. Tanaka E, Kimoto T, Tsuyuguchi K, et al. Effect of clarithromycin regimen for Mycobacterium avium complex pulmonary disease. Am J Respir Crit Care Med. 1999. 160:866–872.
45. Johanson WG Jr, Nicholson DP. Pulmonary disease due to Mycobacterium kansasii: an analysis of some factors affecting prognosis. Am Rev Respir Dis. 1969. 99:73–85.
46. Christensen EE, Dietz GW, Ahn CH, Chapman JS, Murry RC, Hurst GA. Radiographic manifestations of pulmonary Mycobacterium kansasii infections. AJR. 1978. 131:985–993.
47. Evans AJ, Crisp AJ, Hubbard RB, Colville A, Evans SA, Johnston ID. Pulmonary Mycobacterium kansasii infection: comparison of radiological appearances with pulmonary tuberculosis. Thorax. 1996. 51:1243–1247.
48. Ahn CH, Lowell JR, Ahn SS, Ahn SI, Hurst GA. Short-course chemotherapy for pulmonary disease caused by Mycobacterium kansasii. Am Rev Respir Dis. 1983. 128:1048–1050.
49. Griffith DE, Girard WM, Wallace RJ Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria: an analysis of 154 patients. Am Rev Respir Dis. 1993. 147:1271–1278.
50. Wallace RJ Jr, Swenson JM, Silcox VA, Good RC, Tschen JA, Stone MS. Spectrum of disease due to rapidly growing mycobacteria. Rev Infect Dis. 1983. 5:657–679.
51. Wallace RJ Jr. Diagnostic and therapeutic consideration in patients with pulmonary disease due to the rapidly growing mycobacteria. Semin Respir Infect. 1986. 1:230–233.
52. Hazelton TR, Newell JD Jr, Cook JL, Huitt GA, Lynch DA. CT findings in 14 patients with Mycobacterium chelonae pulmonary infection. AJR. 2000. 175:413–416.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download