1. Aisen AM, Chenevert TL. MR spectroscopy : clinical perspective. Radiology. 1989. 173:593–599.
2. Bottomley PA. Human in-vivo NMR spectroscopy in diagnostic medicine : Clinical tool or research probe? Radiology. 1989. 170:1–15.
3. Merchant TE. MR spectroscopy of the breast. Magn Reson Imaging Clin N Am. 1994. 2:691–703.
4. Yoon K-H, Lee M-G, Lee JH, Kim CG, Auh YH. In-vivo 31P magnetic resonance spectroscopy in liver cirrhosis : assessment of phosphorus metabolites according to hepatic dysfunction. J Korean Radiol Soc. 1998. 39:1171–1176.
5. Oberhaensli RD, Hilton-Jones D, Bore PJ, et al. Biochemical investigation of human tumors in vivo with phosphorus-31 magnetic resonance spectroscopy. Lancet. 1986. 2(8497):8–11.
6. Weinreb JC, Newstead G. MR imaging of the breast. Radiology. 1995. 196:593–610.
7. Kaplan O, Cohen JS. Metabolism of breast cancer cells as revealed by non-invasive magnetic resonance spectroscopy studies. Breast Cancer Res Treat. 1994. 31:285–299.
8. Kvistad KA, Bakken IJ, Gribbestad IS, et al. Characterization of neoplastic and normal human breast tissues with in-vivo 1H MR spectroscopy. J Magn Reson Imaging. 1999. 10:159–164.
9. Mackinnon WB, Barry PA, Malycha PL, et al. Fine-needle biopsy specimens of benign breast lesions distinguished from invasive cancer ex vivo with proton MR spectroscopy. Radiology. 1997. 204:661–666.
10. Roebuck JR, Cecil KM, Schnall MD, Lenkinski RE. Human breast lesions: Characterization with proton MR spectroscopy. Radiology. 1998. 209:269–275.
11. Gribbestad IS, Singstad TE, Nilsen G, et al. In-vivo 1H MRS of normal breast and breast tumors using a dedicated double breast coil. J Magn Reson Imaging. 1998. 8:1191–1197.
12. Gribbestad IS, Petersen SB, Fjosne HE, Kvinnsland S, Krane J. 1H NMR spectroscopic characterization of perchloric acid extracts from breast carcinomas and non-involved breast tissue. NMR Biomed. 1994. 7:181–194.
13. Merchant TE, Thelissen GRP, Graaf PW, Otter WD, Glonek T. Clinical magnetic resonance spectroscopy of human breast disease. Invest Radiol. 1991. 26:1053–1059.
14. Kalra R, Wade KE, Hands L, et al. Phosphomonoester is associated with proliferation in human breast cancer : a 31P MRS study. Br J Cancer. 1993. 67:1145–1153.
15. Sijen PE, Wijrdeman HK, Moerland MA, et al. Human breast cancer in vivo:H-1 and P-31 MR spectroscopy at 1.5T. Radiology. 1988. 169:615–620.
16. Twelves CJ, Porter DA, Lowry M, et al. Phosphorus-31 metabolism of post-menopausal breast cancer studied in vivo by magnetic resonance spectroscopy. Br J Cancer. 1994. 69:1151–1156.
17. Leach MO, Verrill M, Glaholm J, et al. Measurements of human breast cancer using magnetic resonance spectroscopy : a review of clinical measurements and a report of localized 31P measurements of response to treatment. NMR Biomed. 1998. 11:314–340.
18. Redmond OM, Stack JP, O'Conner NG, et al. In-vivo Phosphorus-31 magnetic resonance spectroscopy of normal and pathological breast tissues. Br J Radiol. 1991. 64:210–216.
19. Smith TAD, Bush C, Jameson C, et al. Phospholipid metabolites, prognosis and proliferation in human breast carcinoma. NMR Biomed. 1993. 6:318–323.
20. Degani H, Furman E, Fields S. Magnetic resonance imaging and spectroscopy of MCF7 human breast cancer:pathophysiology and monitoring of treatment. Clin Chim Acta. 1994. 228:19–33.
21. Singer S, Souza K, Thilly WG. Pyruvate utilization, phosphocholine and adenosine triphosphate(ATP) are markers of human breast tumor progression: a 31P- and 13C-nuclear magnetic resonance( NMR) spectroscopy study. Cancer Res. 1995. 55(22):5140–5145.
22. Merchant TE, Meneses P, Gierke LW, Otter WD, Glonek T. 31P Magnetic resonance phospholipid profiles of neoplastic human breast tissues. Br J Cancer. 1991. 63:693–698.
23. Twelves CJ, Lowry M, Porter DA, et al. Phosphorus-31 metabolism of human breast - an in-vivo magnetic resonance spectroscopic study at 1.5T. Br J Radiol. 1994. 67:36–45.
24. Degani H, Horowitz A, Itzchak Y. Breast tumors:evaluation with P-31 MR spectroscopy. Radiology. 1986. 161:53–55.
25. Merchant TE, Gierke LW, Meneses P, Glonek T. 31P magnetic resonance spectroscopic profiles of neoplastic human breast tissues. Cancer Res. 1988. 48:5112–5118.
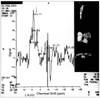
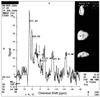
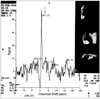


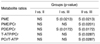





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download