Abstract
Objective
Atrophy and a high T2 signal of the hippocampus are known to be the principal MR imaging findings of hippocampal sclerosis. The purpose of this study was to determine whether or not individual MRI findings correlate with surgical outcome in patients with this condition.
Materials and Methods
Preoperative MR imaging findings in 57 consecutive patients with pathologically-proven hippocampal sclerosis who underwent anterior temporal lobectomy and were followed-up for 24 months or more were retrospectively reviewed, and the results were compared with the postsurgical outcome (Engel classification). The MR images included routine sagittal T1-weighted and axial T2-weighted spin-echo images, and oblique coronal T1-weighted 3D gradient-echo and T2-weighted 2D fast spin-echo images obtained on either a 1.5 T or 1.0 T unit. The images were visually evaluated by two neuroradiologists blinded to the outcome; their focus was the presence or absence of atrophy and a high T2 hippocampal signal.
Results
Hippocampal atrophy was seen in 96% of cases (55/57) [100% (53/53) of the good outcome group (Engel class I and II), and 50% (2/4) of the poor outcome group (class III and IV)]. A high T2 hippocampal signal was seen in 61% of cases (35/57) [62% (33/53) of the good outcome group and 50% (2/4) of the poor outcome group]. All 35 patients with a high T2 signal had hippocampal atrophy. 'Normal' hippocampus, as revealed by MR imaging, occurred in 4% of patients (2/57), both of whom showed a poor outcome (Engel class III). The presence or absence of hippocampal atrophy correlated well with surgical outcome (p<0.01). High T2 signal intensity did not, however, significantly correlate with surgical outcome (p>0.05).
Hippocampal sclerosis, also known as Ammon's horn sclerosis or mesial temporal sclerosis, is the most common disorder associated with medically intractable temporal lobe epilepsy (1-3). Hippocampal sclerosis describes an entity of neuronal loss with associated gliosis involving the hippocampal formation (which we refer to as the hippocampus). Surgical resection of this and the anterior temporal lobe can cure epilepsy in as many as 90% of patients with hippocampal sclerosis, and it is thus imperative that imaging techniques accurately reveal this disorder.
MR imaging has dramatically changed our ability to identify hippocampal sclerosis before surgery (4-6). The hallmark of the condition, as seen on MR images, is an atrophic hippocampus associated with a hyperintense signal on long-repetition-time sequences confined to the hippocampus (4-12). These findings, atrophy and T2 hyperintensity of the hippocampus, are often referred to as the two primary MR imaging findings of hippocampal sclerosis. Patients with these primary MR findings have a 70% to 90% probability of being free of seizures after temporal lobectomy (4, 13). On the other hand, if there are no primary MR findings, the patient has a less than 50% likelihood of becoming seizure-free after surgery. Few reports, however, have addressed the question of which of these primary MR findings shows greater correlation with surgical outcome (4). The purpose of this study was to provide an answer to this.
Fifty-seven consecutive patients with pathologically-proven hippocampal sclerosis who underwent anterior temporal lobectomy between October 1994 and April 1997 for the treatment of intractable medial temporal lobe epilepsy and were followed-up for over 24 months were included in this study. Thirty-eight were male and 19 were female, and their a mean age 33 (range, 20 to 55) years. Surgical candidates were determined on the basis of concordance among the various noninvasive examinations including clinical evaluation, scalp EEG, MR imaging, ictal video-EEG monitoring using scalp electrodes, neuropsychologic evaluation, interictal and ictal SPECT, and PET and/or the intracarotid amobarbital (Amytal) test (Wada's test). In a few selected patients with either 'normal' MR imaging findings or discordance between MR imaging and PET and/or ictal SPECT, invasive intracranial subdural EEG study was performed. A pathologic diagnosis of hippocampal sclerosis was made qualitatively by an experienced neuropathologist without the aid of hippocampal neuronal counting.
Standard MR images were obtained using a 1.0 T or a 1.5 T unit, with conventional spin-echo T1-weighted sagittal and T2-weighted axial and coronal imaging procedures. Slice thickness/gap was 5 mm/1 mm. In addition, 3-mm-thick T2-weighted fast spin-echo imaging and 1.5-mm-thick T1-weighted three-dimensional magnetization preparation rapid acquisition with gradient echo (MPRAGE) imaging of the temporal lobes were performed in the oblique coronal plane. The angle of oblique coronal imaging was perpendicular to the long axis of the hippocampus, resulting in slight variation of angulation from patient to patient. Spatial resolution was approximately 1.0 mm × 1.0 mm (matrix size 256 × 256, FOV 25 cm).
All preoperative MR images were reviewed retrospectively by two neuroradiologists blinded to the postsurgical outcome. Their visual interpretation focused on the presence or absence of hippocampal atrophy and increased T2 signal of the hippocampus. Hippocampal atrophy was deemed to have occurred if one side of the hippocampus was asymmetrically small. This was best appreciated in oblique coronal images and characterized by a reduction in vertical and horizontal cross-sectional diameters. Depending on whether one side of the hippocampus appeared smaller than the opposite side by 50% or more (Fig. 1), or by less than 50% (Fig. 2), hippocampal atrophy was arbitrarily graded as severe or mild. Equivocal findings of atrophy and of high T2 signal were resolved by consensus of the two neuroradiologists.
At 24 months or more follow-up, postoperative outcome was classified by an epileptologist according to Engel's four categories (13). Class I (seizure-free) includes those who have been seizure-free since surgery (or have experienced only auras, atypical generalized seizures occurring when antiepileptic medication is withdrawn), as well as those who enjoyed two years of seizure-free remission up to the most recent follow-up. Class II indicates rare seizures; that is, a few seizures in a year. Class III denotes worthwhile improvement, meaning at least a 75% improvement in seizure frequency compared with preoperative status, while class IV indicates no worthwhile improvement.
Following analysis of the MR imaging findings and postsurgical outcome, individual MR imaging findings of hippocampal atrophy and high T2 hippocampal signal were correlated with postsurgical outcome. Engel class I and II were regarded as good outcome, and class III and IV as poor outcome. Fisher's exact test provided univariate analysis.
Good outcome was found in 93% of patients (53/57); 48 were Engel class I and five were class II. The remaining 7% (4/57) were in the poor outcome group; three were class III and one was class IV.
Hippocampal atrophy was seen in 96% of patients (55/57), and was severe in 33 and mild in 22. The remaining two showed no atrophy. A high T2 hippocampal signal was detected in 61% of patients (35/57), all of whom had combined atrophy. Hippocampal atrophy only, without a high T2 signal, was noted in 35% (20/57). No patient showed a high T2 signal only, nor did MR imaging reveal any case of bilateral hippocampal sclerosis.
Of 55 patients with hippocampal atrophy, 53 (96%) had a good outcome, while only two (4%) had a poor outcome. Hippocampal atrophy was seen in 100% (48/48) (Fig. 1) and 100% (5/5) of patients in Engel class I and II groups, respectively, but in only 50% (2/4) of those with a poor outcome (Engel class III and IV) (Fig. 2). The presence or absence of hippocampal atrophy showed significant correlation with surgical outcome (p<0.01).
In the Engel class I group, severe atrophy was seen in 58% of cases (28/48) and mild atrophy in 42% (20/48). In the Engel class II group, all five patients showed severe atrophy, but this was observed in neither of the class III patients (Fig. 3). One patient in class IV showed mild atrophy and a high T2 hippocampal signal.
All the 33 patients with severe atrophy showed a good outcome (Engel class I and II); of the 22 with mild atrophy, 20 (91%) showed a good outcome and only two (9%) had a poor outcome. There was no significant difference in outcome between the severe and mild atrophy groups (p>0.05). The relationship between MR imaging findings of hippocampal atrophy and surgical outcome is set out in Table 1.
Of 35 patients with high T2 signal intensity, 33 (94%) showed a good outcome and in only two (6%) was the outcome poor. A high T2 signal was seen in 58% (28/48) and 100%(5/5) of Engel class I and II patients, respectively, but in 50% (2/4) of the poor outcome group (Engel class III and IV). Of 22 patients who showed no high T2 hippocampal signal, 20 (91%) with only hippocampal atrophy had a good outcome (Engel class I), whereas two (9%) with an apparently normal hippocampus had a poor outcome. The presence or absence of a high T2 signal did not significantly affect outcome (p>0.05). The relationship between a high T2 hippocampal signal, as revealed by MR imaging, and surgical outcome is seen in Table 2.
In hippocampal sclerosis, atrophy of the hippocampus is the most common and consistent MR imaging finding. In such patients, significant correlation between hippocampal size, as determined by MR imaging, and hippocampal neuronal density has been observed (10, 19, 21). In surgical specimens of 11 patients with cryptogenic temporal lobe epilepsy, Bronen et al. (19) found a statistically significant correlation between hippocampal size, as measured by MR imaging, and neuronal density in CA3 and CA4 of the cornu ammonis and the granular cell layer of the hippocampus.
Our results indicated that in the preoperative evaluation of candidates for surgery among patients with hippocampal sclerosis, an MR imaging finding of hippocampal atrophy is the most useful sole prognostic indicator. Although quantitative hippocampal volumetry has been shown to predict postsurgical outcome (4), we demonstrated that the interpretation of MR images by visual inspection alone has a similar prognostic value. Specifically, hippocampal atrophy, when apparent on visual inspection, independently predicted a good outcome. It has been suggested that a finding of hippocampal atrophy is more useful than one of high T2 signal (4), and this is the case in the present study. Indeed, we noted hippocampal atrophy more often than high T2 signal (97% vs. 61%), and 20 patients with hippocampal atrophy but no such signal showed a good outcome. The severity of atrophy did not, however, show significant correlation with surgical outcome, a finding which disagreed with the conclusions of Jack et al. (4). The reason for this disagreement is uncertain, but might be due to different evaluation methods: qualitative visual inspection in our study and quantitative volumetry in theirs.
Jack et al. (4) reported a statistically significant correlation between computer-assisted measurement of hippocampal volume (atrophy) and surgical outcome (i.e. postoperative seizure control) in 50 patients. In their study, 34 (97%) of 35 patients in whom hippocampal atrophy and EEG concordantly lateralized the focus of seizure had a satisfactory outcome, whereas in eight (53%) of the remaining 15 with either no hippocampal atrophy or hippocampal atrophy discordant with EEG, the outcome was satisfactory. In their prospective study using visual interpretation, Kuzniecky et al. (17) reported that as indicators of surgical success, abnormal MR images had an 82% predictive value and normal images had a 56% predictive value. They suggested that the best predictor of favorable surgical outcome was hippocampal atrophy (p<0.09).
Increased hippocampal T2 signal, reflecting gliosis, is another important indicator of hippocampal sclerosis (7, 9, 22). The degree and extent of hippocampal gliosis also correlate with the T2 signal in the hippocampus (22, 23). Kuzniecky et al. (22) found a high signal in 71% of patients with severe gliosis and in 50% of those with moderate gliosis. They stated that in most cases the severity of pathologic change corresponded to the intensity of the abnormal signals seen on T2-weighted images. In some cases, however, no agreement was found, and the location of the abnormal signal intensity did not always correlate with the distribution of observed histologic change (22). Previous MR imaging studies have described a variable frequency of T2 signal change in the hippocampus: change was observed in 12% to 65% of patients with hippocampal sclerosis (6, 8, 19). In the present study, the increased signal observed on T2-weighted images occurred in 61% of cases. Because it detected the high signal intensity of hippocampal sclerosis more frequently than did the usual T2-weighted sequence, Jack et al. (24) recommended the routine use of the fluid-attenuated inversion recovery (FLAIR) pulse sequence in patients with temporal lobe epilepsy. If the FLAIR sequence had been used in this study, a high hippocampal signal would probably have been detected in more patients; at the time of the study the sequence was not available however.
Garcia et al. (18) reported that in a prospective study involving visual inspection, both ipsilateral hippocampal atrophy and increased ipsilateral hippocampal signal independently predicted a seizure-free outcome. In the present study, however, for reasons which are uncertain, high T2 signal intensity did not significantly correlate with surgical outcome (p>0.05).
In this study, the causes of poor outcome seen in four patients are not known; in two patients whose MR imaging findings appeared normal, PET, ictal EEG and the findings of invasive intracranial EEG suggested a lateralizing focus and led to surgery. In the other two patients, MR imaging findings of mild atrophy and a high T2 hippocampal signal were concordant with the findings of PET and/or ictal EEG. In all four patients, pathologic examination revealed hippocampal sclerosis. The poor outcome in these cases might be because postsurgical scierotic tissue remnants were more widespred.
In conclusion, of the two primary MR imaging findings of hippocampal sclerosis, hippocampal atrophy is more common and shows closer correlation with surgical outcome than does a high T2 signal. It therefore appears to be the more useful predictor of surgical outcome in patients with hippocampal sclerosis, though the reason for the strong correlation between hippocampal atrophy and outcome is not certain.
Figures and Tables
Fig. 1
Example of severe hippocampal atrophy.
Oblique coronal T2-weighted MR image shows shows severe atrophy, with increased signal intensity in right hippocampus (arrow). The patient's outcome was Engel class I.
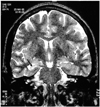
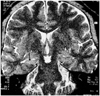
Fig. 2
Example of mild hippocampal atrophy.
Oblique coronal T2-weighted MR image shows mild atrophy, with increased signal intensity in right hippocampus (arrow). The patient's outcome was Engel class IV.
References
1. Babb TL, Brown WJ. Engel J, editor. Pathological findings in epilepsy. Surgical Treatment of the Epilepsies. 1987. New York, NY: Raven Press;511–540.
2. Bruton CJ. The Neuropathology of Temporal Lobe Epilepsy. 1988. Oxford: Oxford University Press.
3. Falconer MA. Mesial temporal (Ammon's horn) sclerosis as a common cause of epilepsy: aetiology, treatment, and prevention. Lancet. 1974. 2:767–770.
4. Jack CR Jr, Sharbrough FW, Cascino GD, et al. Magnetic resonance image-based hippocampal volumetry: correlation with outcome after temporal lobectomy. Ann Neurol. 1992. 31:138–146.
5. Jack CR. Epilepsy: surgery and imaging. Radiology. 1993. 189:635–646.
6. Bronen RA. Epilepsy: the role of MR imaging. AJR. 1992. 159:1165–1174.
7. Tien RD, Felsberg GJ, Castro C, et al. Complex partial seizures and mesial temporal sclerosis: evaluation with fast spin-echo MR imaging. Radiology. 1993. 189:835–842.
8. Jackson GD, Berkovic SF, Tress BM, et al. Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990. 40:1869–1875.
9. Jackson GD, Berkovic SF, Duncan JS, Connelly A. Optimizing the diagnosis of hippocampal sclerosis using MR imaging. AJNR. 1993. 14:753–762.
10. Casino GD, Jack CR Jr, Parisi JE, et al. Magnetic resonance imaging-based volume studies in temporal lobe epilepsy: pathological correlations. Ann Neurol. 1991. 30:31–36.
11. Berkovic SF, Andermann F, Oliver A, et al. Hippocampal sclerosis in temporal lobe epilepsy demonstrated by magnetic resonance imaging. Ann Neurol. 1991. 29:175–182.
12. Bronen RA, Fulbright RK, Spencer DD, et al. Refractory epilepsy: comparision of MR imaging, CT, and histopathologic findings in 117 patients. Radiology. 1996. 201:97–105.
13. Engel J Jr. Engel J, editor. Outcome with respect to epileptic seizures. Surgical Treatment of the Epilepsies. 1987. New York, NY: Raven Press;553–571.
14. Earle KM, Baldwin M, Penfield W. Incisural sclerosis and temporal lobe seizures produced by hippocampal herniation at birth. Arch Neurol Psychiatry. 1953. 69:27–42.
15. Duncan R, Sagar HJ. Seizure characteristics, pathology and outcome after temporal lobectomy. Neurology. 1987. 37:405–409.
16. Meldrum BS, Vigouroux RA, Brierley JB. Systemic factors and epileptic brain damage. Arch Neurol. 1973. 29:82–87.
17. Kuzniecky R, Burgard S, Faught E, Morawetz R, Bartolucci A. Predictive value of magnetic resonance imaging in temporal lobe epilepsy surgery. Arch Neurol. 1993. 50:65–69.
18. Garcia PA, Laxer KD, Barbaro NM, Dillon WP. Prognostic value of qualitative magnetic resonance imaging of hippocampal abnormalities in patients undergoing temporal lobectomy for medically refractory seizures. Epilepsia. 1994. 35:520–524.
19. Bronen RA, Cheung G, Charles JT, et al. Imaging findings in hippocampal sclerosis: correlation with pathology. AJNR. 1991. 12:933–940.
20. Nayel MH, Awad IA, Luders H. Extent of mesiobasal resection determines outcome after temporal lobectomy for intractable complex partial seizures. Neurosurgery. 1991. 29:55–61.
21. Lencz T, McCarthy G, Bronen RA, et al. Quantitative magnetic resonance imaging in temporal lobe epilepsy: relationship to neuropathology and neuropsychological function. Ann Neurol. 1992. 31:629–637.
22. Kuzniecky R, de la Sayette V, Ethier R, et al. Magnetic resonance imaging in temporal lobe epilepsy: pathologic correlations. Ann Neurol. 1987. 22:341–347.
23. Van Paesschen W, Revesz T, Duncan JS, et al. Quantitative neuropathology and quantitative MRI of hippocampal sclerosis. Epilepsia. 1994. 35:S8. 19.
24. Jack CR Jr, Rydberg CH, Krecke KN, et al. Mesial temporal sclerosis: diagnosis with fluid-attenuated inversion-recovery versus spin-echo MR imaging. Radiology. 1996. 199:367–373.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download