Abstract
In stenosis of a segmental branch or among multiple renal arteries, Doppler sampling of intrarenal arteries in the upper, mid and lower poles demonstrates strikingly different waveform patterns that might otherwise be overlooked. We report a case of segmental branch renal artery stenosis in which a pulsus parvus et tardus waveform was observed in a segmental branch of a renal artery. In this case, systematic analysis of Doppler waveforms of intrarenal arteries at more than three different locations facilitated a rapid and confident diagnosis of segmental branch renal artery stenosis.
Renal artery stenosis (RAS) is the commonest correctable cause of hypertension, and in hypertensive patients, duplex ultrasonography (US) is used to screen for renovascular hypertension (1). Efforts to detect renal artery stenosis originally focused on direct scanning of the stenotic segment of a main renal artery, but in practice it is very difficult to evaluate this segment directly. To overcome this problem, an indirect approach in which Doppler waveforms are obtained from intrarenal arteries has therefore been used more frequently.
Segmental branch renal artery stenosis can be a cause of renovascular hypertension, and in one large angiographic series was found to occur in 11% of patients (2). The sequential sampling of multiple intrarenal arteries may detect segmental branch renal artery stenosis, but to our knowledge, the ultrasonographic diagnosis of this condition has not been described in the radiologic literature. We report a case of segmental branch renal artery stenosis in which the analysis of significantly different Doppler waveforms of intrarenal arteries in the upper, mid and lower poles of the kidney permitted rapid, confident diagnosis.
A 27-year-old woman in whom hypertension had first been noted two months earlier presented with intermittent claudication, which had affected her for eight months. Physical examination on admission revealed normal vital signs except for a blood pressure of 200/110 mmHg and weak arterial pulses in the right lower extremity. On auscultation, carotid and abdominal bruits were audible. Laboratory testing revealed normal serum electrolyte, blood urea nitrogen and serum creatinine levels, and urinalysis findings were unremarkable: urine collected at 24 hours and tested for vanillylmandelic acid, catecholamines and 17-ketosteroids was normal.
To determine whether vascular disease was present, imaging studies including Doppler US and aortography with selective renal arteriography were performed. For Doppler examination, a real-time unit (Ultramark 9; Advanced Technology Laboratories, Bothell, Wash., U.S.A.) was used, with a low wall filter (25Hz), low pulse-repetition frequency (2,500Hz), a transducer frequency of 3.5MHz, power output of 63 (85%), and sample volume of 3.5 mm. Renal US showed that both kidneys were of normal length, measuring 10.5 cm on the right side and 10.2 cm on the left, and that their cortical echogenicity was normal. Doppler US of intrarenal arterial branches in the upper and mid poles of the left kidney demonstrated normal waveforms (Fig. 1A), but in the lower pole, abnormally small and prolonged Doppler waveforms were noted, suggestive of upstream renal artery stenosis or occlusion (Fig. 1B). The resistive index from the lower pole of the left kidney was lower (range, 0.40-0.45) than those obtained from the upper and mid poles (range, 0.56-0.65), and on the basis of these Doppler US findings, lower pole segmental artery stenosis was suspected.
Arch aortography demonstrated multiple irregular luminal narrowing in its main branches; abdominal aortography showed mild luminal irregularity in the abdominal aorta and segmental narrowing of the right external iliac artery, the cause of the intermittent claudication. Both main renal arteries were intact, and on each side there was one only; on the left, severe luminal narrowing was noted in a lower segmental branch (Fig. 2A). Selective left renal angiography depicted a severe (>90%) stenotic lesion in a segmental branch supplying the lower pole of the left kidney, and a further such lesion (more than 50% stenosis) in a segmental branch of the anterior division of the left renal artery (Fig. 2B). Occurring as they did in this young female patient, these angiographic findings were highly suggestive of Takayasu's arteritis. During hospitalization she was treated with antihypertensive and immunosuppresive agents, and at the time of discharge, her blood pressure was 130/80 mmHg.
RAS is the commonest cause of secondary hypertension and may lead to renal ischemia, with resultant renal failure (1). Because renal revascularization may cure or significantly improve high blood pressure or renal function, the identification of RAS is important. Conventional angiography remains the standard modality for determining the presence and extent of RAS, but because it is costly and invasive, is inappropriate for screening for this condition. MR/CT angiography or captopril scanning is a useful non-invasive alternative.
Since it is a simple, noninvasive technique and potentially provides physiologic information regarding the hemodynamic status of the kidney, Doppler US is also used to screen for renovascular hypertension (3), and many studies have evaluated its usefulness in the detection of RAS. Early attempts to detect this condition focused on Doppler spectral changes occurring in the stenotic segment of the main renal artery (4-6), but in practice, complete evaluation of this artery may be prevented by obesity, bowel gas, respiratory motion, transmitted aortic pulsation, and difficulty in measuring the Doppler angle.
To overcome the problems and limitations associated with the direct scanning of main renal arteries, several Doppler criteria pertaining to the spectral analysis of intrarenal arteries have been developed for the diagnosis of RAS. Using this intrarenal approach, Doppler US has, in this respect, a fairly high technical success rate, short examination time, and high sensitivity and specificity (7, 8). There is, however, controversy as to whether the intrarenal vessel investigated should be central or peripheral. Schwerk et al. (7) proposed that attention be focused on interlobar rather than segmental arteries, but Eibenberger et al. (8), on the basis of an experimental study involving animals, reported that Doppler spectra were more reliable if obtained from segmental rather than interlobar arteries. In those reports, segmental arteries were defined as those lying within the renal sinus, and interlobar arteries as those within the renal parenchyma, as depicted by US.
Hemodynamically significant stenosis of a renal artery causes changes in the shape of the time-velocity waveform in the downstream arterial network. There is generally delayed or prolonged early systolic acceleration and diminished amplitude and rounding of a systolic peak, features which have been described as the classic signs of pulsus parvus et tardus (a small and prolonged pulse) (4). Some authors have described methods for measuring systolic acceleration, the so-called systolic acceleration index (AI) and acceleration time (AT) (3, 4, 9). Using this approach, Stavros et al. (9) proposed that hemodynamically meaningful RAS (>60%) was best achieved using a prolonged acceleration time (greater than 0.07) and a diminished acceleration index (less than 3.0 m/sec2). Because it is influenced not only by vascular impedance downstream but also by hemodynamically substantial RAS upstream, renal resistive index (RI), the Doppler parameter most commonly used to determine the status of renal hemodynamics, can also be used for the diagnosis of RAS (4, 7). Although there is considerable overlap in RI measurements between normal and stenotic arteries, an RI of significantly lower value (e.g. less than 0.56) may be abnormal, and in a case involving substantial unilateral RAS, the difference in mean RI between the two kidneys may be significant (7).
Doppler US diagnosis is much more complicated and difficult in a case of accessory or segmental branch RAS than in RAS of a main artery. Although the condition most commonly involves the proximal main renal artery, and accessory or segmental branch RAS generally accompanies stenosis of a main artery, focal stenosis limited to an accessory renal artery or segmental branch can also be a cause of renovascular hypertension. In one large angiographic series, segmental branch RAS was reported to occur in 11% of patients (2). Accessory renal arteries are common, occurring in approximately 20% of kidneys (10, 11), but because insonation of these arteries is often impossible-even with color Doppler US-stenosis which may be present is likely to be overlooked. Also, if stenosis occurs only in a segmental artery, a normal Doppler waveform produced by nonstenotic branches will lead to a false-negative result. To minimize the possibility that RAS in an accessory renal artery or segmental branch is overlooked, spectral analysis should therefore involve multiple regions including one upper pole, one mid pole, and one lower pole in each kidney. In this respect our case is interesting because it demonstrates that segmental branch RAS can be easily diagnosed by detecting significantly different Doppler waveforms among multiple areas of the kidney.
Severe stenosis upstream from the artery causes a pressure drop just distal to the stenosis sufficiently severe to cause pulsus parvus et tardus in the downstream arterial network. However, the Doppler sign is not specific to stenosis of the main renal artery but indicates narrowing of any artery upstream to the sampled intrarenal arteries. A segmentally isolated dampened waveform in one kidney may indicate hemodynamically significant stenosis of a branch vessel or accessory renal artery rather than of a main renal artery. When a waveform of this kind occurs, it is important to exclude the possibility that the result is false positive. If the tracing is reproducible, Doppler angle correction is appropriate, and if there is no adjacent cyst or mass that might dampen the arterial waveform, it is reasonable to suspect segmental branch or accessory RAS and mandatory to confirm this by conventional angiography. Renin determination in a segmental renal vein or captopril renography may be used to assess the functional significance of segmental disease secondary to stenosis of a branch vessel or accessory renal artery (12, 13).
In stenosis of a segmental branch or among multiple renal arteries, Doppler sampling of intrarenal arteries in the upper, mid and lower poles reveals strikingly different waveform patterns that might otherwise be overlooked. When a segmentally isolated pulsus parvus et tardus waveform is observed, this may be an important indicator of hemodynamically significant stenosis of a segmental branch or accessory renal artery, which is often a correctable cause of hypertension.
Figures and Tables
 | Fig. 1Spectral Doppler studies at intrarenal arteries of the left kidney.
A. Longitudinal sonogram of this kidney shows a normal appearance. Doppler sample volume was placed in a distal segmental artery or proximal interlobar artery in the mid pole. Doppler tracings show a very abrupt rise to peak systolic velocity. The RI value was 0.6.
B. Doppler tracings at an intrarenal artery in the lower pole demonstrate slowed systolic upsweep and dampened maximum systolic Doppler shift. Note decreased slope during early systolic upsweep. The waveform is less pulsatile than that seen in A. The RI value was 0.45.
|
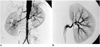 | Fig. 2Digital subtraction angiography in the same patient.
A. Aortogram shows mild luminal irregularity in the abdominal aorta. The main renal arteries are intact and single on each side. A severely narrowed segment (black arrow) is noted in a segmental branch of the left renal artery.
B. Selective left renal arteriogram shows a stenotic lesion (black arrow) in the posterior division of the left renal artery (more than 90% stenosis). A further stenotic lesion (white arrow) is seen in a segmental branch of the anterior division of the left renal artery (more than 50% stenosis).
|
Acknowledgments
This study was supported by Seoul National University Hospital Research Fund (02-1997-349-0).
References
1. Working group on Renovascular Hypertension. Detection, evaluation, and treatment of renovascular hypertension. Arch Intern Med. 1987. 147:820.
2. Bookstein JJ. Segmental renal artery stenosis in renovascular hypertension. Radiology. 1968. 90:1073–1083.
3. Handa N, Fukunaga R, Etani H, et al. Efficacy of echo-Doppler examination for the evaluation of renovascular disease. Ultrasound Med Biol. 1988. 14:1–5.
4. Patriquin HB, Lafortune M, Jequier JC, et al. Stenosis of the renal artery: assessment of slowed systole in the downstream circulation with Doppler sonography. Radiology. 1992. 184:479–485.
5. Avasthi PS, Voyles WF, Greene ER. Noninvasive diagnosis of renal artery stenosis by echo-Doppler velocimetry. Kidney Int. 1984. 25:824–829.
6. Taylor DC, Kettler MD, Moneta GL, et al. Duplex ultrasound scanning in the diagnosis of renal artery stenosis: a prospective evaluation. J Vasc Surg. 1988. 7:363–369.
7. Schwerk WB, Restrepo IK, Stellwaag M, et al. Renal artery stenosis: Grading with image-directed Doppler US evaluation of renal resistive index. Radiology. 1994. 190:785–790.
8. Eibenberger K, Schima H, Trubel W, et al. Intrarenal Doppler ultrasonography: which vessel should be investigated? J Ultrasound Med. 1995. 14:451–455.
9. Stavros AT, Parker SH, Yakes WF, et al. Segmental stenosis of the renal artery: pattern recognition of tardus and parvus abnormalities with duplex sonography. Radiology. 1992. 184:487–492.
10. Desberg AL, Paushter DM, Lammert GK, et al. Renal artery stenosis: evaluation with color Doppler flow imaging. Radiology. 1990. 177:749–753.
11. Berland LL, Koslin DB, Routh WD, et al. Renal artery stenosis: prospective evaluation of diagnosis with color duplex US compared with angiography. Radiology. 1990. 174:421–423.
12. Lee SM, Drach GW. Renovascular hypertension from segmental renal artery stenosis: importance of segmental renal vein renin sampling. J Urol. 1980. 124:704–706.
13. Katial R, Ziessman HA. Segmental branch renal artery stenosis diagnosed with captopril renography. J Nucl Med. 1992. 33:266–268.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download