Abstract
Extracranial carotid artery dissection may manifest as arterial stenosis or occlusion, or as dissecting aneurysm formation. Anticoagulation and/or antiplatelet therapy is the first-line treatment, but because it is effective and less invasive than other procedures, endovascular treatment of carotid artery dissection has recently attracted interest. We encountered two consecutive cases of trauma-related extracranial internal carotid artery dissection, one in the suprabulbar portion and one in the subpetrosal portion. We managed the patient with suprabulbar dissection using a self-expandable metallic stent and managed the patient with subpetrosal dissection using a balloon-expandable metallic stent. In both patients the dissecting aneurysm disappeared, and at follow-up improved luminal patency was observed.
Extracranial carotid artery dissection usually occurs following blunt trauma but can also occur spontaneously, especially in patients with fibromuscular dysplasia or connective tissue disease (1). It may manifest as arterial stenosis or occlusion in cases subintimal dissection, or as dissecting aneurysm formation in cases of subadventitial dissection (1). Arterial stenosis and/or aneurysm may lead to artery-to-artery embolism, and or hemodynamic insufficiency may sometimes cause watershed infarction (2). Initial management is focused mainly on the prevention of thromboembolism. Anticoagulation with heparinization and/or antiplatelet therapy is the first-line treatment, but when dissections of the carotid artery are either symptomatic, are asymptomatic without resolution, or occur in patients in whom long-term anticoagulation treatment is contraindicated, surgical therapy - even though technically demanding - has been recommended (3). Because it is effective and less invasive than surgery, endovascular treatment of carotid artery dissection has, however, recently attracted interest, and some case reports have described endovascular techniques using stents that allow the preservation of arterial patency (4-11).
We encountered two consecutive cases of trauma-related extracranial internal carotid artery dissection, one in the suprabulbar portion and one in the subpetrosal portion of this artery. We describe the clinical and angiographic features of both cases and the ways in which these lesions were managed. We also present the follow-up results.
A 40-year-old, previously healthy man presented with left hemiparesis and left facial weakness which had developed four hours before admission after being struck on the right side of the neck by the edge of a door. No external wound was observed. CT and MRI of the brain revealed acute infarction in the vascular territory of the right middle cerebral artery. Intravenous heparinization was initiated, thus maintaining a twice-normal activated partial thromboplastin time (aPTT). Angiography performed on admission revealed near-complete occlusion of the suprabulbar portion of the right internal carotid artery and complete occlusion of the ipsilateral proximal middle cerebral artery, suggesting dissection of the vessel and subsequent arterial thromboembolism (Figs. 1A, 1B). On the sixth day following admission the patient's mental status deteriorated suddenly, and CT revealed a large intracerebral hemorrhage within the territory of the infarcted right middle cerebral artery. Intravenous heparinization was discontinued and the hematoma was evacuated. Follow-up angiography at day 20 revealed partial restoration of luminal patency; the lumen, however, was largely compromised by the presence of a thick intimal flap (Fig. 1C). Because of the risk of continuing anticoagulation therapy, endovascular treatment of the dissection was planned.
Two days before angioplasty, performed at day 35, daily doses of aspirin (100 mg) and ticlopidine (250 mg) were initiated and continued thereafter. A 9-Fr guiding catheter was introduced into the common carotid artery. A heparin bolus of 5000 IU was injected intravenously, and in order to maintain aPTT at 1.5 to 2 times normal, was continued for one day. Atropine (1mg) was injected intravenously just before stent placement; for primary stenting of the dissected segment, a self-expandable uncovered metallic stent 30 mm in length and 8 mm in diameter (Easy Wallstent; Boston Scientific Corporation, Watertown, Mass., U.S.A.) was used (Fig. 1d), and for postdilatation, a 6-mm diameter angioplasty catheter (Ultra-thin; Boston Scientific Corporation, Watertown, Mass., U.S.A.) at a low inflation pressure was employed. Intermittent neurological examinations were conducted throughout the procedure, and after stenting, focal residual dissected false lumen at the posterior portion of the artery was observed (Fig. 1e). Interestingly, the symptom of left side weakness showed slight improvement two days after stent placement, motor power having increased from grade 2 to 3.
Thirty-six days after the procedure, follow-up axial CT scanning and 2D reconstruction images showed complete reconstitution of the arterial lumen and disappearance of the false lumen. Ticlopidine was discontinued.
A 39-year-old man presented with multiple fractures of the lower extremities and a fractured left zygoma and skull base after a motor vehicle accident. Brain CT performed immediately after arrival in the emergency room showed no significant abnormality and orthopedic management was initiated. On the fourth day following admission, however, the patient showed mild weakness of the right extremities, and brain CT revealed cortical areas of low density at the left frontal and occipital lobes. Angiography was performed the following day, and focal but significant stenosis associated with a dissecting aneurysm was observed at the subpetrosal portion of the left internal carotid artery (Fig. 2A). Because the patient was not in a fit condition for sustained anticoagulation therapy, percutaneous angioplasty aimed at reestablishing the vessel lumen was performed during the same session. A 7-Fr guiding catheter was introduced into the proximal portion of the internal carotid artery, and to prevent thromboembolic complications a heparin bolus of 5000 IU was administered intravenously. For primary stenting of the affected portion of the vessel, a premounted balloon-expandable uncovered stent (Nir Primo; Boston Scientific Scimed, Inc., Maple Grove, Minn., U.S.A.) was used. This was 16 mm in length and was dilated to 4.2 mm with a balloon at a pressure of 10 atm (Fig. 2B). A postangioplastic angiogram showed improved luminal patency, with reduction of the aneurysmal sac (Fig. 2C). Aspirin (100 mg/day) and ticlopidine (250 mg/day) were subsequently administered, and to maintain aPTT at 1.5 to 2 times normal, [JW2]heparinization was continued for five days after stenting. Follow-up angiography performed 65 days after stent placement showed good luminal patency. Although the filled aneurysmal sac was significantly smaller, focal filling of a part of it was noted (Fig. 2D). Ticlopidine was discontinued. The patient's symptom was improved gradually after the procedure, and the motor power of the affected extremities returned to normal at the time of follow-up angiography.
Carotid dissection is initiated by hemorrhage into the medial layer of the artery due to various causes. Where the dissection primarily involves the subintima, stenosis or occlusion of the artery usually results, and angiography reveals the so-called 'string sign'. If the subadventitia is principally involved, an out-pouching sac, a dissecting aneurysm in the form of an outponihing sae usually arises. Stenosis and aneurysm may also appear in combination. Luminal stenosis can cause distal ischemia leading to stroke or transient ischemic attacks. In both subintimal and subadventitial dissections the resulting exposure of the basement membrane leads to platelet aggregation, and this serves as an embolic source (1). It has been suggested that ischemic symptoms are more often secondary to embolic phenomena than to hypoperfusion caused by stenosis (2).
Because many cases escape diagnosis or patients receive some form of therapy, the natural history of carotid dissection remains somewhat unclear. Although its clinical course may be benign (1), overall mortality rates have been consistently reported at 20-40% (3). In addition, in a recent study of the time course of symptoms in extracranial carotid artery dissection, the authors observed ischemic events in 82.5% of afflicted patients and complete stroke in two-thirds (12). These results imply that the time course of carotid dissection is not at all benign, and the same authors emphasized the need for early preventive measures to avoid ischemic complications (12).
The management goal in cases of extracranial carotid dissection is to prevent the development or worsening of neurologic deficits by preventing thrombosis and embolization (3): options include observation, anticoagulation therapy, surgery, and endovascular treatment. Fabian et al. suggested that anticoagulative measures should be used in all non-occlusive arterial dissections without contraindications, regardless of the degree of luminal narrowing (13). Anticoagulation is often initially achieved with heparin and then, after 1-3 weeks, maintained with warfarin, which is continued for up to 6 months. Surgical repair is reserved for the management of symptomatic patients with lesions in an accessible location (3), but the technique for the preservation of the artery is technically demanding and time consuming. For many years, endovascular occlusion of the affected artery has involved the use of detachable balloons or coils as an alternative to surgery. The advent of stenting techniques in carotid artery angioplasty has, however, led to their exploitation in the preservation of arterial patency. The techniques are much easier and safer than surgical repair, and published case reports have described their successful application (4-11).
Various stents can be used for the restoration of luminal patency and occlusion of the sac of a dissecting aneurysm: some authors have insisted that a covered stent is especially useful (11), though stents without fabric covering have also been used for the same purpose with successful results, as in our cases. To obliterate dissecting aneurysms, Guglielmi detachable coils along with stents have also been used (10). For suprabulbar internal carotid artery dissection we applied a self-expandable stent, and for subpetrosal internal carotid artery dissection, the balloon-expandable type: in both cases the results were successful. Simionato et al. emphasized the superiority of self-expandable stents over the balloon-expandable type in terms of their radial expansile force and longitudinal flexibility (5). They surmised that the first of these two factors was helpful for effectively sealing off the dissecting aneurysm, and in a case involving the use of a balloon-expandable stent the aneurysm sac also effectively disappeared. The balloon-expandable stent we used was also flexible, a feature which allowed us to place it in an angled portion of the vessel. Follow-up showed that the softness of the stent did not compromise luminal patency of the vessel. Selection of a stent must be individualized on the basis of factors which include the characteristics and location of the lesion and the diameter and geometry of the vessel.
During the management of case 1, we delayed stent placement because of an infarction in the patient's right middle cerebral artery territory due to embolic occlusion. Where there is overt parenchymal infarction, as in that patient, determining the optimal timing of endovascular recanalization and anticoagulation can be an issue. For the stabilization of already-infarcted parenchyma to which anticoagulative measures have been applied, a wait of three to four weeks is recommended, though in case 1 there was unexpected hemorrhagic transformation of the infarction. Because flow through the patent anterior communication artery was good, management could have involved occlusion of the dissected ICA: because of the relative ease and safety of the endovascular recanalization methods now available, however, a destructive method of this kind was not considered.
In case 2, the cause of the left cerebral cortical infarction was not clear. Because a dissecting aneurysm is already known to be a potential embolic source, the dissected segment was recanalized after initial angiography without evaluating the significance of the stenosis. Angiography appeared to indicate that this was narrow enough to cause a watershed infarction due to hemodynamic insufficiency, and that stenting of the stenotic segment could recanalize the stenosis as well as seal the aneurysmal sac. Follow-up angiography, however, revealed a focal residual sac. suggesting that the dissection had opened: in such cases spontaneous closure is possible, as in the case described by Simionato et al. (5).
In conclusion, endovascular stent placement is a safe and effective method for the management of patients with extracranial carotid artery dissections with or without associated aneurysm.
Figures and Tables
Fig. 1
Case 1
A. Lateral angiogram of right common carotid artery shows occlusion of its proximal internal branch. Focal dimpling (black arrow) of the posterior aspect indicates partial filling of false lumen by thrombus (small arrows). Distal true lumen has collapsed, with linear filling by contrast media.
B. Left internal carotid angiogram shows complete occlusion of proximal segment of right middle cerebral artery, suggesting thromboembolic occlusion.
C. Follow-up angiogram obtained 20 days after initial angiography shows partial recanalization of true lumen. A thick dissecting flap is present (arrows) and the dilated false lumen persists.
D. Angioplasty was performed 35 days after injury, and a self-expandable metallic stent is seen.
E. After angioplasty using a stent, true lumen regained its original diameter. The false lumen, now smaller, may be observed behind the stent. Thirty-five days after stent placement, patency was assessed with CT and the false lumen was no longer visible (not shown).
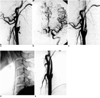
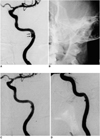
Fig. 2
Case 2
A. Initial angiogram of left internal carotid artery demonstrates a focal stenosis of the subpetrosal portion of the artery, associated with a dissecting aneurysm (arrows). The aneurysmal sac is restricted by the bony canal of that portion.
B. A balloon-expandable stent was inserted, but is barely visible due to the relatively poor radiopacity and surrounding bony structures.
C. Luminal patency of the dissected segment is improved substantially after stent placement, and the aneurysmal sac is much smaller.
D. Follow-up angiography was performed 65 days after stent placement. The arterial lumen is well preserved, though the aneurysmal sac is still partly filled. After stent placement, the patient was asymptomatic.
References
1. Anson J, Crowell RM. Cervicocranial arterial dissection. Neurosurgery. 1991. 29:89–96.
2. Lucas C, Moulin T, Deplanque D, et al. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. 1998. 29:2646–2648.
3. Mulloy JP, Flick PA, Gold RE. Blunt carotid injury: a review. Radiology. 1998. 207:571–585.
4. Bejjani GK, Monsein LH, Laird JR, et al. Treatment of symptomatic cervical carotid dissections with endovascular stents. Neurosurgery. 1999. 44:755–760.
5. Simionato F, Righi C, Scotti G. Post-traumatic dissecting aneurysm of extracranial internal carotid artery: endovascular treatment with stenting. Neuroradiology. 1999. 41:543–547.
6. DeOcampo J, Brillman J, Levy DI. Stenting: a new approach to carotid dissection. J Neuroimaging. 1997. 7:187–190.
7. Bernstein SM, Coldwell DM, Prall JA, Brega KE. Treatment of traumatic carotid pseudoaneurysm with endovascular stent placement. J Vasc Interv Radiol. 1997. 8:1065–1068.
8. Coric D, Wilson JA, Regan JD, Bell A. Primary stenting of the extracranial internal carotid artery in a patient with multiple cervical dissections: technical case report. Neurosurgery. 1998. 43:956–959.
9. Horowitz MB, Miller G III, Meyer Y, Carstens G III, Purdy PD. Use of intravascular stents in the treatment of internal carotid and extracranial vertebral artery pseudoaneurysms. AJNR. 1996. 17:693–696.
10. Klein GE, Szolar DH, Raith J, et al. Post-traumatic extracranial aneurysm of the internal carotid artery: combined endovascular treatment with coils and stents. AJNR. 1997. 18:1261–1264.
11. Chalmers RT, Brittenden J, Bradbury AW. The use of endovascular stented grafts in the management of traumatic false aneurysms: a caveat. J Vasc Surg. 1995. 22:337–338.
12. Biousse V, D'Anglejan-Chatillon J, Touboul P, Amarenco P, Bousser M. Time course of symptoms in extracranial carotid artery dissections. Stroke. 1995. 26:235–239.
13. Fabian TC, Patton JH Jr, Croce MA, et al. Blunt carotid injury: importance of early diagnosis and anticoagulation therapy. Ann Surg. 1996. 223:513–525.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download