Abstract
Objective
To investigate the efficacy of gadobenate dimeglumine (Gd-BOPTA) enhanced MR imaging for the detection of liver lesions in patients with primary malignant hepatic neoplasms.
Materials and Methods
Thirty-one patients with histologically proven primary malignancy of the liver were evaluated before and after administration of Gd-BOPTA at dose 0.05 or 0.10 mmol/kg. T1-weighted spin echo (T1W-SE) and gradient echo (T1W-GRE) images were evaluated for lesion number, location, size and confidence by three off-site independent reviewers and the findings were compared to reference standard imaging (intraoperative ultrasound, computed tomography during arterial portography or lipiodol computed tomography). Results were analyzed for significance using a two-sided McNemar's test.
Results
More lesions were identified on Gd-BOPTA enhanced images than on unenhanced images and there was no significant difference in lesion detection between either concentration. The largest benefit was in detection of lesions under 1 cm in size (7 to 21, 9 to 15, 16 to 18 for reviewers A, B, C respectively). In 68% of the patients with more than one lesion, Gd-BOPTA increased the number of lesions detected.
Early detection of primary malignant tumors of the liver primarily hepatocellular carcinoma and cholangiocarcinoma remains a diagnostic challenge. Ultrasonography (US), contrast enhanced computed tomography (CT) and magnetic resonance (MR) imaging have shown suboptimal results particularly in the setting of chronic liver disease (1-5). Although it has been hypothesized that tissue-specific contrast agents will improve the sensitivity of MR imaging for liver lesion detection, to date only a few studies have reported results with hepatocyte directed contrast agents (6-10). In this study we have investigated the value of gadobenate dimeglumine (Gd-BOPTA) enhanced MR imaging in liver lesion detection based on an off-site evaluation of images in patients with proven primary hepatic malignant neoplasms.
In a Phase II multicenter double-blind study of gadobenate dimeglumine (Gd-BOPTA; Bracco SpA, Milan, Italy), 113 adult patients with known or suspected liver masses were examined. From this group, 31 patients with biopsy proven malignant primary liver tumors (hepatocellular carcinoma in 29 and cholangiocarcinoma in 2) were identified. There were 8 women and 23 men with a mean age of 59 years (range 32 to 75 years). In 14 patients there was biopsy or imaging evidence of cirrhosis.
Based on a reference standard imaging, a total of 71 focal lesions were identified in these patients. The presence and absence of a liver lesion was confirmed by intra operative ultrasound (IOUS) in 24 patients (48 lesions), computed tomography during arterial portography (CTAP) in 4 patients (15 lesions), and lipiodol CT (CTLP) in 3 patients (8 lesions). All patients received at least one of these reference standard imaging studies of the liver within 7 days before to 14 days after the MR imaging. In patients with more than one reference standard, IOUS was preferred with CTAP being the next preferred examination. A cyst and two focal areas of focal nodular hyperplasia were identified and excluded from further analysis.
Gadobenate dimeglumine is an octadentate chelate of gadolinium which may be used as a non-specific contrast agent like the conventional gadolinium agents as well as a liver-specific contrast agent (11). The chemical and pharmacodynamic properties of this contrast agent have been described previously (12-16). In brief, following rapid (2 ml/min) intravenous injection of the contrast agent, it initially redistributes into the extracellular space and is then largely eliminated by the kidneys. Some 3-5% of the injected dose is however taken up by functioning hepatocytes and undergoes biliary excretion. Despite the low percentage of hepatobiliary excretion, the fraction taken up produces clinically useful hepatic enhancement that increases liver signal-to-noise ratio (SNR) and liver-to-lesion contrast-to-noise ratio (CNR) (6-8, 14). Peak enhancement of lesion-to-liver contrast occurs between 40 and 120 minutes. Since one goal of the clinical trial was to determine optimal dose, one of two different doses were injected in a blinded fashion. The contrast agent (either 0.25 mmol/L or 0.5 mmol/L solution) was administered intravenously at 10 ml/min immediately after the unenhanced MR examination. Seventeen patients received 0.05 mmol/kg, and 14 patients received 0.10 mmol/kg. Post contrast imaging was performed at two delayed time points in order to determine the optimal imaging period in the hepatocyte phase.
MR imaging was performed at 1.5 Tesla with T1-weighted spin echo (SE) and T1-weighted gradient echo (GRE) pulse sequences before contrast administration as well as 40-80 minutes (early) and 90-120 minutes (late) after contrast administration. The liver was imaged with 10-14 axial 10 mm thick sections with 2 mm gaps; a matrix size of 140-160×256, and a tailored field of view ranging from 285-350×380-400 mm. T1-weighted SE sequences utilized a repetition time between 350-550 msec and an echo time of 15 msec and 3-4 excitations. The T1-weighted GRE images were acquired with a repetition time of 95-150 msec, a 4 msec echo time, an 800 flip angle and one excitation during a single breath hold. T2 weighted images were also obtained but were not included in the analysis.
Three off-site independent reviewers (A, B, C) evaluated the MR images separately in a random unpaired fashion that included the pre contrast images and the post contrast images. Each set of MR images was evaluated individually and the number, size, and location by surgical segment of lesions were recorded on liver maps. Additionally, each reviewer, using a 5-point scale system, documented the confidence with which a lesion was detected. A point of 1 indicated that the lesion was definitely present, a point of 2 indicated that a lesion was probably present while a point of 3 indicated an indeterminate lesion. Point of 4 and 5 indicated that a lesion was probably not present or definitely not present respectively.
Each reviewer assessed the presence of lesions for each patient at each time point for each sequence. The total number of lesions as well as their size and location was determined from the reference standard imaging. In a patient with multiple lesions, no more than five lesions per patient were assessed in order not to bias the findings toward patients with multiple lesions. Subsequently, the five largest lesions from the reference standard imaging were analyzed. This allowed inclusion of all patients without skewing the data from the patients with many lesions. A lesion was considered present if the reviewer confidence ranking was either a 1 or 2 point (definitely or probably present). False positive lesions were defined as lesions reported by the reviewers with a confidence of 1 or 2 with no corresponding lesion by the reference standard. Analysis according to lesion size was performed by dividing the lesions into three groups based on diameter size: 1 cm or less; between 1 cm to 2 cm; and greater than 2 cm.
The efficacy of gadobenate dimeglumine enhanced MRI was evaluated by assessing the change in detection (relative to the reference standard) from pre-contrast MRI to contrast enhanced MRI. The change in concordance with the reference standard from unenhanced to enhanced MRI in the determination of the number of focal liver lesions was tested using a two tailed McNemars test. A p-value of 0.05 or less was considered statistically significant. Differences in concordance were also studied between concentrations and imaging times using a two tailed McNemars test. Because all patients were selected on the basis of lesion pathology, specificity and accuracy could not be assessed.
The number of focal liver lesions identified on Gd-BOPTA enhanced MR images exceeded the number identified on unenhanced images (Table 1). Table 1 separates the number of lesions identified by reviewer by time point as well as by imaging sequence. By combining both the T1-weighted GRE and T1-weighted SE images before and after contrast (both early and late time points) for reviewers A, B and C, the number of lesions identified increased after contrast administration from 34 to 53, 37 to 48, and 43 to 49, respectively. This difference was statistically significant for 2 of the 3 reviewers (p<0.05). There was no significant difference in lesion detection between the two concentrations (0.05 mmol/kg and 0.10 mmol/kg) of contrast administered. Table 1 shows that, in general, more lesions were identified with the T1-weighted GRE pulse sequence when compared to the T1-weighted SE technique, and more lesions were identified after contrast administration when both imaging techniques were analyzed together. Most lesions were identified on the late imaging time point (90-120 minutes) although several more lesions (total number) were identified combining both the early and late post-contrast images.
The number of false positive lesions decreased for two of the three reviewers after the administration of Gd-BOPTA (Table 2). There were 15 patients with solitary lesions. One reviewer detected all of these lesions while the other two reviewers detected 14 out of 15 solitary lesions. The administration of contrast was not needed to identify any of these solitary lesions. There were 5 patients with 2 lesions; both lesions were identified only after contrast administration in 4 of the 5 patients. In the remaining patient with two lesions, the second lesion (25 mm) was not identified before or after contrast. In two of these patients gadobenate dimeglumine administration allowed identification of the second lesion while in the other two patients, Gd-BOPTA administration was required for the visualization of both (Fig. 1). There were 3 patients with 3 lesions. One lesion was identified before and after gadobenate dimeglumine in all of these patients; however, the second and third lesions were only identified by one of the reviewers in one patient and two reviewers in another patient. In the third patient with three lesions, one reviewer detected the second and third lesion only without contrast administration. There were 3 patients with 4 lesions and 5 patients with 5 lesions. Two out of three reviewers detected more lesions after contrast administration in 6 out of these 8 patients (Fig 2). In the remaining two patients, one patient had no additional lesions identified while only 1 out of the 3 reviewers detected more lesions after contrast in the other patient. In 68% of the patients with more than one lesion by reference standard (11 of 16 patients), more lesions were identified by 2 of the 3 reviewers on contrast enhanced images.
After contrast administration, smaller lesions were better detected by two of the three reviewers (Table 3). From a total of 29 lesions smaller than 1 cm, the number of lesions detected before and after contrast infusion for reviewers A, B, and C where 7 to 21, 9 to 15, and 16 to 18 respectively. This was statistically significant (p<0.05) for only two of the three reviewers (Fig 3). The number of lesions detected for both the 1-2 cm and greater than 2 cm group also increased after contrast administration.
This study shows that gadobenate dimeglumine enhanced T1-weighted MR imaging can improve the detection of primary hepatic malignant neoplasms. Our findings confirm prior reports that have noted improvement in lesion-liver contrast on Gd-BOPTA enhanced images. Furthermore, our findings show that the most important benefit is in the detection of small (less than 1 cm) tumors.
Prior reports have provided conflicting findings on contrast enhanced MR imaging using hepatocyte targeted contrast agents in patients with cirrhosis. For example, Murakami et al. noted that compared to normal liver, patients with cirrhosis had lower hepatic enhancement with Mn-DPDP (9) while Manfredi et al. reported similar enhancement between normal and cirrhotic livers with gadobenate dimeglumine (6). These differences may be due to variations in the severity of cirrhosis in the subject groups or due to differences in pharmacokinetics of the two drugs as Gd-BOPTA also shows non-specific tissue enhancement (extracellular effect) in addition to hepatocyte specific liver enhancement. The differentiation of the hepatic neoplasm may also be important.
Our results have important implications with respect to imaging technique and the evaluation of patients with primary hepatic neoplasms. Firstly T1-weighted GRE techniques were superior compared to SE techniques and there was only minimal improvement in the total number of lesions detected when both sequences were used together. Prior studies have in fact reported that quantitative measurement of liver-to-lesion contrast on T1-weighted GRE images is superior to that on SE images (17). This advantage is most likely related to greater T1-weighting on the GRE images due to the availability of shorter TEs and to decreased motion-related noise in these breath-hold images. Thus liver imaging with gadobenate dimeglumine should be performed with short TE GRE techniques. These images can also be obtained at thinner section (8 mm) with ample coverage to study the entire liver. While use of fatsaturation may also provide additional benefit, this was not studied and requires future attention (18).
In terms of the optimal imaging window, it appears there is a favorable distinction between liver and primary malignant hepatic neoplasms at both the early and late time points after gadobenate dimeglumine administration although slightly more total lesions were detected at the late time point (90-120 minutes after injection). There was no significant difference in lesion detection between the two doses (0.05 mmol/kg and 0.10 mmol/kg) evaluated. One possible way to image with this agent is to initially perform dynamic imaging immediately following the pre-contrast examination (not performed in these cases) and then, if needed, have the patient return at a convenient time between 1-2 hours later to have delayed scans performed. This approach may not only increase the yield in lesion detection in patients with cirrhosis where dynamic scanning is known to be of critical importance, but may also provide important perfusion data for tissue characterization. Moreover, this approach may well be the preferred procedure in patients suspected of harboring liver metastases. In such patients, dynamic imaging with conventional gadolinium agents does not necessarily improve lesion detectability over unenhanced scans alone (19-20).
There are several limitations to our study. The population studied was limited because only patients with known neoplasms were evaluated. This did not allow determination of accuracy, sensitivity or specificity since there was an unintentional bias as the reviewers expected to identify at least one hepatic mass. In addition the off-site analysis methodology may also have biased the results against Gd-BOPTA since clinical information may have improved diagnostic performance was not available. Furthermore, the presence of cirrhosis was not proven in all subjects; it is likely that this patient population represented a spectrum of underlying chronic liver disease and extrapolation to a cirrhotic population should be made with caution. Another limitation was that even though histological diagnosis was available for all the patients, not every lesion was sampled. Finally, comparison of this agent to contrast-enhanced helical CT may also be necessary to determine the clinical utility of gadobenate dimeglumine enhanced liver MR imaging. Clinical utility may also be affected by the fact that the T2-weighted images were not utilized for the evaluation of the images. This methodology was adopted in order to best evaluate the effect of the contrast agent on T1-weighted images. However, in actual practice, patients would not be evaluated without simultaneous review of the T2-weighted images.
In conclusion, Gd-BOPTA may serve a role as a contrast agent to identify primary malignant hepatic neoplasms with MR imaging. From our blinded off-site multicenter evaluation, the contrast agent aids in the detection of smaller lesions in a patient group where traditional imaging (US, CT, MR) has been limited.
Figures and Tables
Fig. 1
61 year-old man with hepatocellular carcinoma.
A. T1-weighted GRE image without contrast demonstrates a small segment 8 lesion (arrow).
B. T1-weighted GRE image after injection of 0.1 mmol/kg of gadobenate dimeglumine shows increased conspicuity of the segment 8 lesion (arrow).
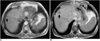
Fig. 2
72 year-old woman with hepatocellular carcinoma.
A. T1-weighted SE image before contrast demonstrates a large lesion within segment 2-3.
B. T1-weighted SE image performed 90 minutes after injection of 0.05 mmol/kg of gadobenate dimeglumine better delineates a second lesion within segment 4 (arrow). Note even though there is enhancement of the tumor, the lesion to liver contrast still increases.
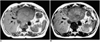
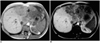
Fig. 3
35 year-old woman with hepatocellular carcinoma.
A. T1-weighted GRE image before contrast demonstrates large primary lesion.
B. T1-weighted GRE image performed 90 minutes after injection of 0.05 mmol/kg of gadobenate dimeglumine better identifies the large primary tumor but also of smaller lesions within the right lobe.
References
1. Miller WJ, Baron RL, Dodd GD III, Federle MP. Malignancies in patients with cirrhosis: CT sensitivity in 200 consecutive transplant patients. Radiology. 1994. 193:645–650.
2. Dodd GD III, Miller WJ, Baron RL, Skolnick ML, Campbell WL. Detection of malignant tumors in end-stage cirrhotic livers: Efficacy of sonography as a screening technique. AJR. 1992. 159:727–733.
3. Nelson RC, Chezmar JL, Sugarbaker PH, Bernadino ME. Hepatic tumors: Comparison of CT during arterial portography, delayed CT, and MR imaging for preoperative evaluation. Radiology. 1989. 171:47–51.
4. Baron RL. Understanding and optimizing use of contrast material for CT of the liver. AJR. 1994. 163:323–331.
5. Taourel PG, Pageaux GP, Coste V, et al. Small hepatocellular carcinoma in patients undergoing liver transplantation: detection with CT after injection of iodized oil. Radiology. 1995. 197:377–380.
6. Manfredi R, Maresca G, Baron RL, et al. Gadobenate Dimeglumine [BOPTA] enhanced MR imaging: Patterns of enhancement in normal liver and cirrhosis. J Magn Reson Imaging. 1998. 8:862–867.
7. Caudana R, Morana G, Pirovano GP, et al. Focal malignant hepatic lesions: MR imaging enhanced with gadolinium Benzyloxypropoinictetra- acetate (BOPTA)- Preliminary results of phase II clinical application. Radiology. 1996. 199:513–520.
8. Vogl TJ, Stupavsky A, Pegios W, et al. Hepatocellular carcinoma: Evaluation with dynamic and static Gadobenate Dimeglumine-enhanced MR imaging and histopathologic correlation. Radiology. 1997. 205:721–728.
9. Murakami T, Baron RL, Peterson MS, et al. Hepatocellular carcinoma: MR imaging with Mangafodipir Trisodium. Radiology. 1996. 200:69–77.
10. Petersein J, Spinazzi A, Giovagnoni A, et al. Focal liver lesions: evaluation of the efficacy of gadobenate dimeglumine in MR imaging- a multicenter phase III clinical study. Radiology. 2000. 215:727–736.
11. Kirchin MA, Pirovano G, Spinazzi A. Gadobenate dimeglumine (Gd-BOPTA): an overview. Invest Radiol. 1999. 33:798–809.
12. Vittadini G, Felder E, Musu C, Tirone C. Preclincial profile of Gd-BOPTA; a liver specific MRI contrast agent. Invest Radiol. 1990. 25:S59–S60.
13. De Haen C, Gozzini L. Soluble-type hepatobiliary contrast agents for MR imaging. J Magn Reson Imaging. 1993. 3:179–183.
14. Spinazzi A, Lorusso V, Pirovano G, Taroni P, Kirchin M, Davies A. MultiHance clinical pharmacology: biodistribution and MR enhancement of the liver. Acad Radiol. 1998. 5:S. 86–89.
15. Spinazzi A, Lorusso V, Pirovano G, Kirchin M. Safety, tolerability, biodistribution and MR enhancement of the liver with Gd-BOPTA: results of clinical pharmacology and pilot imaging studies in non-patient and patient volunteers. Acad Radiol. 1999. 6:282–291.
16. Vogl TJ, Pegios W, McMahon C, Balzer J, et al. Gadobenate Dimeglumine- a new contrast agent for MR imaging: Preliminary evaluation in healthy volunteers. AJR. 1992. 158:887–892.
17. Rosati G, Pirovano G, Spinazzi A. Interim results of phase II clinical testing of Gadobenate Dimeglumine. Invest Radiol. 1994. 29:S. S183–S185.
18. Slater GJ, Saini S, Mayo-Smith W, Sharma P, et al. Mn-MPDP enhanced MR imaging of the liver: analysis of pulse sequence performance. Clin Radiol. 1996. 51:484–486.
19. Peterson MS, Baron RL, Murakami T. Hepatic malignancies: usefulness of acquisition of multiple arterial and portal venous phase images at dynamic gadolinium-enhanced MR imaging. Radiology. 1996. 201:337–345.
20. Hamm B, Mahfouz AE, Taupitz M, et al. Liver metastases: improved detection with dynamic gadolinium-enhanced MR imaging. Radiology. 1997. 202:677–682.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download