Abstract
Objective
To determine the feasibility of transcaval transjugular intrahepatic portosystemic shunt (TIPS) in patients with occluded previous TIPS.
Materials and Methods
Between February 1996 and December 2000 we performed five transcaval TIPS procedures in four patients with recurrent gastric cardiac variceal bleeding. All four had occluded TIPS, which was between the hepatic and portal vein. The interval between initial TIPS placement and revisional procedures with transcaval TIPS varied between three and 31 months; one patient underwent transcaval TIPS twice, with a 31-month interval. After revision of the occluded shunt failed, direct cavoportal puncture at the retrohepatic segment of the IVC was attempted.
Results
Transcaval TIPS placement was technically successful in all cases. In three, tractography revealed slight leakage of contrast materials into hepatic subcapsular or subdiaphragmatic pericaval space. There was no evidence of propagation of extravasated contrast materials through the retroperitoneal space or spillage into the peritoneal space. After the tract was dilated by a bare stent, no patient experienced trans-stent bleeding and no serious procedure-related complications occurred. After successful shunt creation, variceal bleeding ceased in all patients.
It has been reported that transjugular intrahepatic portosystemic shunt (TIPS) is a useful procedure in the treatment of patients with uncontrolled variceal hemorrhage or intractable abdominal ascites resulting from portal hypertension (1). It is an interventional procedure leading to decompression of the splanchnic venous system in patients with portal hypertension by creating a low-resistance channel between an intrahepatic branch of the portal vein and a main hepatic vein. Although it has gained wide acceptance for the management of portal hypertension, a number of major problems such as an increased risk of hepatic encephalopathy and a high rate of shunt dysfunction have been associated with the technique (2-8).
Shunt dysfunction is a consequence of shunt stenosis or occlusion, and can lead to recurrent bleeding or ascites. Thus, early detection and correction of shunt dysfunction may improve the secondary patency rate of TIPS. In most patients, TIPS revision is a safe and effective means of restoring shunt patency and achieving symptomatic relief. In some cases, however, TIPS patency cannot be restored in the usual way, and a satisfactory outcome demands aggressive intervention. When standard shunt revision fails and the hepatic vein cannot be catheterized or is too small to create a new shunt, a direct shunt between the retrohepatic inferior vena cava (IVC) and the portal vein can be safely performed. In this article, we report our experiences and evaluate the feasibility of transcaval TIPS in patients with failed standard shunt revision.
Between February 1996 and December 2000, 57 consecutive patients with malfunctioning TIPS underwent revision of previous TIPS (n=36) or new TIPS placement (n=21) for recurrent gastric cardiac variceal bleeding. Five transcaval TIPS procedures were subsequently performed in four patients, in all of whom TIPS between the hepatic and portal vein was occluded. In all cases, the indication for initial TIPS was recurrent bleeding from gastric cardiac varices. All patients, who were men aged 49-58 (mean, 53) years, were first referred to our department for revision of a previous shunt. The interval between initial TIPS placement and transcaval TIPS varied between three and 31 months, this latter period representing the interval between two transcaval TIPS procedures performed in one patient. In all cases, portal hypertension was due to hepatic cirrhosis. Three patients had postnecrotic cirrhosis due to hepatitis B viral infection, and in one, cirrhosis due to alcoholic liver disease was present (Table 1). According to the Childs-Pugh classification of hepatocellular disease, at the time of transcaval TIPS this was class A in one, class B in three, and class C in one.
All procedures were performed via the right internal jugular vein, accessed using the standard Seldinger technique. A 9-F long vascular sheath (Cook Inc., Bloomington, U.S.A.) containing a 9-F dilator was advanced into the inferior vena cava, and using a 5-F multipurpose catheter and 0.035-inch hydrophilic guidewire (Terumo, Tokyo, Japan), previous TIPS were then accessed for recanalization. The shunts could not be catheterized, however. After failed attempts to negotiate a guidewire into the occluded lumen, stent puncture using a Colapinto transjugular portal venous access needle was attempted, but this also failed. In addition, there were no appropriate hepatic veins for new TIPS, and since it was thus impossible to catheterize the occluded shunt or hepatic veins for new TIPS creation, transcaval TIPS was attempted.
Direct cavoportal puncture from the intrahepatic segment of the inferior vena cava to the portal vein using a Colapinto transjugular portal venous access needle was attempted. Using the previous TIPS stent as a target, the needle was advanced through the hepatic parenchyma into the portal vein under fluoroscopic guidance. After puncturing the vein, a guidewire was advanced through the needle and manipulated along it and into the splenic vein. The Colapinto needle was then withdrawn and a 5-F multipurpose catheter was passed over the guidewire without dilatation of the parenchymal tract; venography from that position demonstrated large cardiac varices and occlusion of the previous TIPS (Fig. 1). Pressure measurements were obtained, and the portosystemic gradient thus determined. The guidewire was replaced with an Amplatz extra-stiff wire (Cook Inc., Bloomington, U.S.A.), the 9-F sheath with its 9-F dilator was advanced into the portal vein, and the dilator was withdrawn. The sheath was then retracted over the wire from the portal vein to the IVC while simultaneous contrast material injection through a side-arm adapter was performed to confirm the parenchymal location of the tract prior to balloon dilatation (Fig. 2). After evaluating the puncture site, the parenchymal tract was dilated using an Ultrathin Diamond balloon catheter 10 mm in diameter (Medi-Tech/Boston Scientific, Watertown, Mass., U.S.A.) (Fig. 3), and a 10-mm-diameter Wallstent (Schneider USA, Minneapolis, Minn., U.S.A.) was deployed. The stent was then expanded using a 10-mm angioplasty balloon. A 5-F multipurpose catheter was readvanced into the splenic vein; venography from this position was performed and the portosystemic pressure gradient was remeasured.
The patients were discharged from hospital 1-2 weeks after transcaval TIPS and were followed up at 3-month intervals. Doppler ultrasound (US) was performed 3-5 days after TIPS insertion, and thereafter at 3-month intervals.
Transcaval TIPS was successful in all cases. In all patients, 10-mm-diameter stents were positioned after balloon dilatation, and no immediate complications were observed. The portosystemic pressure gradient before transcaval TIPS placement was elevated, and direct portal venography demonstrated prominent gastric cardiac varices. After placement, the mean pressure gradient ranged from 5 to 13 mmHg. Although effective portal vein decompression was achieved, concomitant transcatheter coil embolization of varices was performed in two cases because portal venography demonstrated prominent filling of gastric cardiac varices (Fig. 4). After TIPS placement, bleeding stopped in all cases and during hospitalization did not recur. No patient developed encephalopathy.
In three of five cases, simultaneous contrast injection through a side-arm adapter while the sheath was retracted from the parenchymal tract demonstrated minimal or moderate extravasation of contrast material (Figs. 2, 3). This was, however, confined to hepatic subcapsular space or free space in the pericaval and subdiaphragmatic area, and it did not change shape. There was no evidence of propagation of extravasated contrast materials through the retroperitoneal space or spillage into the peritoneal space. After tract dilatation by deploying a bare stent, no transstent bleeding occurred, and repeated shunt venography demonstrated good flow through the shunt and no further extravasation of contrast material (Fig. 4). No serious procedure-related complications were observed, and blood pressure changed neither during nor after the procedures. There was no evidence of internal bleeding after stent placement, and patients were discharged without incident one week later.
Using Doppler US, shunt patency was followed up in three patients. In two of these, shunts were patent 3 and 25 months after transcaval TIPS placement, respectively, and in the other, Doppler US detected shunt occlusion at 24 months follow-up. In that patient variceal bleeding recurred 31 months after previous transcaval TIPS, and a new, parallel, transcaval TIPS was created (Fig. 2). The previous shunt was positioned through the right portal vein; the subsequent transcaval shunt was successfully positioned through the portal vein near its bifurcation point. Variceal bleeding in that patient resolved after new TIPS. One patient died of progressive hepatic failure 20 months after successful transcaval TIPS placement.
TIPS are functionally equivalent to small-bore portocaval or mesocaval shunts. One of their disadvantages compared to surgically created portosystemic shunts is an increased risk of shunt failure (4-7), the most common cause of which is the development of pseudointimal hyperplasia (8). TIPS stenosis is most commonly located at the hepatic vein insertion site and is less frequently observed within the parenchymal tract (5, 9). Some investigators have speculated that bile extravasation from adjacent transected bile ducts occurring during TIPS (a bile leak into the shunt) may stimulate thrombosis or accelerate the process of pseudointimal hyperplasia within the shunt track and the hepatic vein that results in TIPS stenosis or occlusion (10-12). LaBerge et al. (10) reported bile staining within shunts in several explant specimens, suggesting that an inflammatory response to bile may aggravate the hyperplastic response. In addition, technical error may also be a cause of some TIPS stenosis. Inadequate stent length overlap leaving a bare area of liver parenchyma has been shown to be a cause of shunt occlusion. Stent shortening can also occur over time, causing the metallic stent to recede into the parenchymal tract.
Primary one-year TIPS patency rates have been reported to be 25-66% (5, 13, 14). A two-year incidence of TIPS dysfunction of up to 90% was reported when invasive hemodynamic measurement was performed, though with careful sonographic follow-up and TIPS revision as needed, the secondary patency rate of TIPS is reported to be as high as 92% two years after placement (7).
TIPS revision generally involves angioplasty for stenosis after negotiating the shunt lumen, and more recently has involved the placement of additional stents (12). This allows not only the restoration of TIPS function, but may also modify the configuration of the shunt in a way that prevents future restenosis. Shunt occlusion is found in 13-16% of all patients undergoing revision (5, 8, 15). The recanalization of chronically occluded shunts is technically more difficult than in cases involving a stenosed shunt. If a guidewire can be advanced through the occluded lumen into the portal vein, the occluded shunt can be recanalized by angioplasty with or without coaxial placement of a new stent. When primary guidewire passage through the occluded shunt fails, direct puncture of the lumen of the stent may be attempted. If access to the shunt proves impossible, the creation of an entirely new parallel shunt may be necessary, though in some instances the catheterization of a different hepatic vein may be difficult or impossible. As in our cases, there may be no hepatic veins capable of supporting new TIPS. When the hepatic vein cannot be catheterized, portal vein puncture at the stump of the hepatic vein of the previous TIPS or the intrahepatic segment of the inferior vena cava itself can be attempted.
The concept of a direct portocaval shunt is not new. Haskal et al. (16) described the creation of an intrahepatic portocaval shunt between the IVC and the right portal vein; in their cases the hepatic veins were inadequate because of the cephalad location of the portal vein bifurcation relative to the hepatic veins. The procedure has also been described in a patient who lacked hepatic veins capable of supporting TIPS, in one with Budd-Chiari syndrome, and in another with occluded previous TIPS (16-18). Other possible indications for transcaval portosystemic decompression include the need for a transfemoral approach in which the internal jugular veins or superior vena cava are thrombosed and the inferior right hepatic vein is inadequate for TIPS.
Our cases are examples of portocaval shunt formation in patients with occluded previous TIPS resulting in or accompanying unusable hepatic veins. Most stent stenoses occur at the hepatic venous end (8), and because small hepatic veins are more prone to stenosis, transcaval TIPS can be a solution where the hepatic vein is too small to create TIPS. It is also a means by which secondary or tertiary TIPS can be created, regardless of whether hepatic venous access is possible.
In TIPS, the puncture may on rare occasions enter the portal vein in an extrahepatic location, and if this is not recognized, balloon dilatation of the tract will result in portal vein laceration with significant intraperitoneal bleeding. Under such circumstances, the use of a bare stent to create a shunt that extends into a portal vein segment not enveloped by liver parenchyma can result in potentially fatal intra-abdominal hemorrhage, a complication that can be avoided by careful evaluation of the portal vein location prior to the procedure. The feasibility of direct portocaval shunt also depends on the existence of a safe route between the IVC and the portal veins; such a route is provided when the tract linking these vessels is completely intraparenchymal, a location which must be confirmed. Using the same method as that employed in the evaluation of the anatomic relationship between the hepatic capsule and the portal vein segment, we evaluated the safety of the tract before stent deployment or tract dilatation. This involved pulling back a sheath or catheter over a safety wire and injecting contrast material through a side-arm adapter. In three cases in our series, tractography revealed slight leakage of contrast materials into either hepatic subcapsular or subdiaphragmatic pericaval space. For an extended period, however, the shape of the extravasated contrast materials was unchanged. Because there was no evidence of propagation of contrast materials through the retroperitoneal space, or spillage into peritoneal space, the tract was dilated by deploying a bare stent. Although bridging may be safer with a covered stent than with a bare one, trans-stent bleeding did not occur in our cases; because the shunt exits the retrohepatic IVC at a site that is well invested by the fibrous tissue of the retroperitoneum, such bleeding can be prevented (16).
The retrohepatic inferior vena cava has been measured in cadaveric studies; the average length in the craniocaudal dimension of the retrohepatic segment was found to be 6.7 (range, 3.5-10.9) cm (19, 20), and the vessel was totally enclosed by liver substance in 30% of cases (20). This enclosed hepatic substance had an average height of 3.2 cm and an average thickness of 0.7 cm, and in cases where the IVC was partially exposed, the average thickness of the nonenclosed IVC posterior wall was 1.0 cm. Those studies showed that because the tract is entirely intraparenchymal, ventral wall puncture of the inferior vena cava at this location is in most cases safe. In addition, the extravasation of contrast materials during the transcaval TIPS procedures in our series indicated that the retroperitoneal space surrounding the IVC may be confined and restricted, and invested by fibrous tissue. Although there was no bleeding through the porous mesh at the caval end of the stent in such cases, this hypothesis requires further anatomical investigation.
McCowan et al. (21) reported a case of cardiac perforation and tamponade during TIPS placement. The heart was, presumably, perforated by instrumentation during the initial phase of the procedure, at which point manipulation to gain suitable access to the hepatic vein was performed. To prevent the occurrence of this unusual complication in transcaval TIPS, it is important that the cavoportal puncture is made at the extracardiac and retrohepatic segment of the IVC, and cephalad to the portal vein. For this purpose, the cephalad end of the occluded stent can be used as a reference marker.
The creation of a new shunt becomes increasingly difficult if a previous TIPS cannot be catheterized and there are no hepatic veins capable of supporting a new TIPS. In such instances, direct portocaval shunt creation permits secondary or tertiary procedures. In our own experience, it can be safely performed even if a small leak is found at evaluation of the tract between the IVC and the portal vein. The use of covered stents may improve the safety of the transcaval approach to TIPS in the intrahepatic segment of the IVC and permit the extension of this concept to the creation of a direct shunt between the extrahepatic IVC and the main portal vein.
Although the number of patients in this study was limited, the favorable results of shunt formation indicate that transcaval TIPS placement is an effective and safe alternative treatment in patients with occluded previous TIPS and no hepatic veins suitable for new TIPS.
Figures and Tables
 | Fig. 1A 52-year-old man with postnecrotic liver cirrhosis and variceal bleeding. Portal venogram obtained through splenic vein injection before transcaval TIPS placement shows occluded previous shunt, which was between the right hepatic and posterior segmental branch of the right portal vein (small arrows). Note filling of prominent gastric cardiac varices with gastrorenal shunt (arrow). |
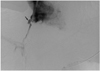 | Fig. 2A 51-year-old man with alcoholic liver cirrhosis who underwent one standard TIPS and two parallel transcaval TIPS.
After transcaval portal vein puncture, contrast material injection through a side-arm adapter of a sheath, with simultaneous retraction of the sheath over the wire, demonstrates slight spillage of contrast material (arrow).
|
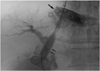 | Fig. 3A 52-year-old man with postnecrotic liver cirrhosis and variceal bleeding. Radiograph demonstrates balloon dilatation of the parenchymal tract (small arrows). Note the extravasation of contrast materials into hepatic subcapsular or subdiaphragmatic pericaval space (arrow). Moderate extravasation of contrast materials is apparent, and an opacified right bile duct is also seen. |
 | Fig. 4A 52-year-old man with postnecrotic liver cirrhosis and variceal bleeding. Portal venogram obtained after transcaval stent placement shows good flow through the stent without opacification of gastric cardiac varices, which were embolized with stainless coils. Trans-stent extravasation of contrast material was not apparent. The postprocedural portosystemic pressure gradient was 5 mmHg. |
References
1. Zemel G, Katzen BT, Becker GJ, et al. Percutaneous transjugular portosystemic shunt. JAMA. 1991. 266:390–393.
2. Sanyal AJ, Freedman AM, Shiffman ML, Purdum PP 3rd, Luketic VA, Cheatham AK. Portosystemic encephalopathy after transjugular intrahepatic portosystemic shunt: results of a prospective controlled study. Hepatology. 1994. 20:46–55.
3. Somberg KA, Riegler JL, LaBerge JM, et al. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunts: incidence and risk factors. Am J Gastroenterol. 1995. 90:549–555.
4. LaBerge JM, Ring EJ, Gordon RL, et al. Creation of transjugular intrahepatic portosystemic shunt (TIPS) with the Wallstent endoprosthesis: results in 100 patients. Radiology. 1993. 187:413–420.
5. Haskal ZJ, Pentecost MJ, Soulen MC, Shlansky-Goldberg RD, Baum RA, Cope C. Transjugular intrahepatic portosystemic shunt stenosis and revision: early and midterm results. AJR. 1994. 163:439–444.
6. Coldwell D, Ring E, Rees C, et al. Multicenter investigation of the role of transjugular intrahepatic portosystemic shunts in the management of portal hypertension. Radiology. 1995. 196:335–340.
7. Saxon RS, Ross PL, Mendel-Hartvig J, et al. Transjugular intrahepatic portosystemic shunt patency and the importance of stenosis location in the development of recurrent symptoms. Radiology. 1998. 207(3):683–693.
8. Sterling KM, Darcy MD. Stenosis of transjugular intrahepatic portosystemic shunts: presentation and management. AJR. 1997. 168:239–244.
9. Hausegger KA, Sternthal HM, Klein GE, Karaic R, Stauber R, Zenker G. Transjugular intrahepatic portosystemic shunt: angiographic follow-up and secondary interventions. Radiology. 1994. 191:177–181.
10. LaBerge JM, Ferrell LD, Ring EJ, Gordon RL. Histopathologic study of stenotic and occluded transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 1993. 4:779–786.
11. Saxon RR, Mendel-Hartvig J, Corless CL, et al. Bile duct injury as a major cause of stenosis and occlusion in TIPS: comparative histologic analysis in humans and swine. J Vasc Interv Radiol. 1996. 7:487–497.
12. Saxon RR, Timmermans H, Uchida BT, et al. Stent grafts for revision of TIPS stenoses and occlusions: A clinical pilot study. J Vasc Interv Radiol. 1997. 8:539–548.
13. Martin M, Zajko AB, Orons PD, et al. Transjugular intrahepatic portosystemic shunt in the management of variceal bleeding: indications and clinical results. Surgery. 1993. 114:719–726.
14. Lind CD, Malisch TW, Chong WK, et al. Incidence of shunt occlusion or stenosis following transjugular intrahepatic portosystemic shunt placement. Gastroenterology. 1994. 106:1277–1283.
15. Nazarian GK, Ferral H, Castaneda-Zuniga WR, et al. Development of stenoses in transjugular intrahepatic portosystemic shunts. Radiology. 1994. 192:231–234.
16. Haskal ZJ, Duszak R, Further EE. Transjugular intrahepatic transcaval portosystemic shunt: the gun-sight approach. J Vasc Interv Radiol. 1996. 7:139–142.
17. Rogopoulos A, Gavelli A, Sakai H, McNamara M, Huguet C. Transjugular intrahepatic portosystemic shunt for Budd-Chiari syndrome after failure of surgical shunting. Arch Surg. 1995. 130:227–228.
18. Soares GM, Murphy TP. Transcaval TIPS: Indications and anatomic considerations. J Vasc Interv Radiol. 1999. 10:1233–1238.
19. Chang RW, Shan-Quan S, Yen WW. An applied anatomical study of the retrohepatic segment of the inferior vena cava. J Anat. 1989. 164:41–47.
20. Camargo AM, Teixeira GG, Ortale JR. Anatomy of the ostia venae hepaticae and the retrohepatic segment of the inferior vena cava, Part 1. J Anat. 1996. 188:59–64.
21. McCowan TC, Hummel MM, Schmucker T, Goertzen TC, Culp WC, Habbe TG. Cardiac perforation and tamponade during transjugular intrahepatic portosystemic shunt placement. Cardiovasc Intervent Radiol. 2000. 23:298–311.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download