Abstract
The authors present a case of giant serpentine aneurysm (a partially thrombosed aneurysm containing tortuous vascular channels with a separate entrance and outflow pathway). Giant serpentine aneurysms form a subgroup of giant intracranial aneurysms, distinct from saccular and fusiform varieties, and in this case, too, the clinical presentation and radiographic features of CT, MR imaging and angiography were distinct.
In 1977 Segal and McLaurin introduced the term 'giant serpentine aneurysm' as a subcategory of giant aneurysms (1). Giant serpentine aneurysms form a subgroup of large intracranial aneurysms that have characteristic computed tomographic (CT), magnetic resonance imaging (MRI) and angiographic features. If unrecognized, they can easily be mistaken for neoplasms, particularly because they often present with progressive neurologic deficits, and CT and MR imaging studies frequently show that they are associated with adjacent edema and mass effect.
Cerebral angiography can establish the presence of a giant serpentine aneurysm and provide the anatomic detail necessary for its treatment. In this report we describe an aneurysm of this kind, located in the middle cerebral artery and with a functioning blood channel passing through the thrombus formed within the aneurysmal sac.
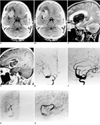
A 50-year-old woman presented with a headache which had started six days earlier. She was neurologically normal and showed neither evidence of language dysfunction nor gait abnormalities. CT scans of the head, with and without contrast enhancement, showed a large mass at the base of the right frontal lobe at the point of distribution of the right middle cerebral artery. Slight calcification was noted in the peripheral portion of the mass (Figs. 1A, 1B). MR images revealed the presence of a giant, partially thrombosed aneurysm with residual flow through a serpiginous lumen within its thrombosed portion (Figs. 1C, 1D). Cerebral angiography demonstrated a partially thrombosed giant aneurysm arising from the right middle cerebral artery. The serpiginous lumen filled slowly and supplied blood to two distal right middle cerebral artery branches arising directly from the lateral aspect of the giant aneurysm (Figs. 1E-1H). These findings were consistent with giant serpentine aneurysm arising at the right middle cerebral artery.
Giant serpentine aneurysms form a subcategory of intracranial giant aneurysms and possess certain characteristic anatomic and radiographic features. A serpentine aneurysm has been defined as a giant, partially thrombosed aneurysm (greater than 2.5 cm in diameter), with tortuous vascular channels that have a separate entrance and outflow pathway (2). The channels may be central or eccentric within the aneurysm, with small branching channels that end blindly. Vascular flow, which supplies distal branches of the cerebral vasculature leading to vital or nonvital areas of the brain, is typically slow. Previous pathologic reports (3, 4) have described these aneurysms as large globoid or pear-shaped masses with a 1.0 to 4.0-mm-thick fibrous wall that may contain numerous small vessels similar to vasa vasorum. Their vascular channels do not seem to be residual lumina of the parent artery but are typically intrathrombotic canals that are not endothelialized and do not contain normal elastic lamina or media. The parent vessel is usually a branch of the middle or posterior cerebral artery, the distal vertebral artery at its junction with the basilar artery, or the supraclinoid internal carotid artery. In our case the serpentine aneurysm involved the right middle cerebral artery and had an irregular channel which continued into normal distal branches.
Giant serpentine aneurysms usually present with an intracranial mass rather than intracranial hemorrhage. A patient's symptoms are dependent on the aneurysm's location, the predominant signs and symptoms being headache, hemiplegia and hemiparesis, visual disturbance, cranial nerve palsy, dysphasia and aphasia, nausea, vomiting, seizure and vertigo.
The radiologic findings of giant serpentine aneurysms are characteristic. Plain-film radiographs may show pineal displacement caused by the mass, curvilinear calcification, and erosive change involving the skull base. CT scans demonstrate an oval-shaped mass of mixed density. On nonenhanced scans, heterogeneous regions of increased attenuation represent thrombus, and tubular regions of decreased attenuation represent a patent vascular channel. After contrast administration, enhancement of the serpentine vascular channel is apparent (5, 6).
The MR imaging findings of giant serpentine aneurysms have not been extensively reported but consist of a mass lesion with a heterogeneous signal that represents various stages of hemoglobin degradation and flow void regions. The aneurysm is clearly separated from normal parenchyma, and the vascular channel, which may be evaluated by phase-contrast MR angiography, may be visible. In our case it was clearly visualized, though its distal small branches were unclear. Conventional angiography is the most powerful means of evaluating the location and state of flow of a giant serpentine aneurysm, the typical features of which were clearly visualized in our case.
The exact cause of giant serpentine aneurysms is unclear. Tomasello et al. (3) reported the five-year progression of a small fusiform aneurysm of a branch of the middle cerebral artery into a giant serpentine aneurysm, suggesting that the latter may have its origin in fusiform aneurysms that grow larger in time and undergo thrombosis and organization. Fodstad et al. (4) described the formation of a giant serpentine aneurysm after carotid ligation for treatment of a giant aneurysm. It has recently been hypothesized that continual growth of a dolichoectatic aneurysm may lead to the formation of a so-called giant serpentine aneurysm (7). Our imaging studies, whose purpose is to illustrate the dynamic nature of one subset of these lesions, demonstrate the dramatic growth of one such partially thrombosed aneurysm from dolichoectatic to serpentine. Because they do not arise at vessel bifurcations or origins of vestigial vessels, lack an anatomic neck, and have separate entrance and exit sites in the vascular channel, we too conclude that giant serpentine aneurysms are not derived from saccular aneurysms.
The treatment of giant serpentine aneurysms should aim to arrest their growth, to eliminate the mass effect, and to obliterate the abnormal vascular channel. The most effective way of accomplishing these goals is the direct and permanent occlusion of the parent artery at the origin of the aneurysm. This can be best achieved by endovascular means: selective catheterization of the parent artery and occlusion of the vessel with detachable balloons, N-butyl cyanoacrylate, or Guglielmi detachable coils. Endovascular occlusion of the parent artery should be performed after careful functional testing of the distal territory to assess any potential neurologic deficit that may ensue. Our patient would not have tolerated endovascular treatment, and she was transferred to another institute.
In conclusion, giant serpentine aneurysms are a specific pathologic entity that can affect the intracranial blood vessels. Their CT, MR and cerebral angiographic features are characteristic. The last mentioned modality is diagnostic and can provide crucial information for the treatment of these lesions, including endovascular occlusion of the parent vessel.
Figures and Tables
Fig. 1
A 50-year-old woman with giant intracranial aneurysm.
A. Precontrast CT scan shows a large oval-shaped mass with increased attenuation representing thrombus (arrows) and slight peripheral calcification (arrowheads) in the right frontal region. Peripheral edema may also be observed.
B. Postcontrast CT scan demonstrates peripheral and central enhancenment. The enhanced central tubular structure represents the serpentine vascular channel (arrow).
C. T2-weighted sagittal image shows a large thrombosed aneurysm (arrows) with peripheral edema (arrowheads) in the right frontal area.
D. Postcontrast T1-weighted sagittal image shows an enhancing tubular structure (arrow) indicating the serpentine vascular channel.
E, F. Angiograms of the right internal carotid artery. Anteroposterior (E) and lateral (F) arterial-phase angiograms show the ectatic vascular channel involving the upper trunk of the right middle cerebral artery and corresponding to the vascular channel identified on MR images.
G, H. Anteroposterior (G) and lateral (H) capillary phase angiograms more clearly demonstrate the tortuous vascular channel of the serpentine aneurysm and show delayed filling of normal cortical branches of the superior division (arrowheads).
References
1. Segal HD, McLaurin RL. Giant serpentine aneurysm: Report of two cases. J Neurosurg. 1977. 46:115–120.
2. Aletich VA, Debrun GM, Monsein LH, Nanta HJW, Spetzler RF. Giant serpentine aneurysms: a review and presentation of five cases. Am J Neuroradiol. 1995. 16:1061–1072.
3. Tomasello F, Albanese V, Cioffi FA. Giant serpentine aneurysms: a separate entity. Surg Neurol. 1979. 12:429–432.
4. Fodstad H, Liliequist B, Wirell S, Nilsson PE, Boquist L, Abdul-Rahman A. Giant serpentine intracranial aneurysm after carotid ligation: case report. J Neurosurg. 1978. 49:903–909.
5. Haddad GF, Haddad FS. Cerebral giant serpentine aneurysm: case report and review of the literature. Neurosurgery. 1988. 23:92–97.
6. Belec L, Cesaro P, Brugieres P, Gray F. Tumor-simulating giant serpentine aneurysm of the posterior cerebral artery. Surg Neurol. 1988. 29:210–215.
7. Vishteh AG, Spetzler RF. Evolution of a dolichoectatic aneurysm into a giant serpentine aneurysm during long-term follow up: case illustration. J Neurosurg. 1999. 91:346.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download