Abstract
Objective
To evaluate the technical feasibility and the clinical effectiveness of sclerotherapy for the treatment of peritoneal inclusion cysts (PICs).
Materials and Methods
Between June 1996 and February 2001, eight PICs in seven female patients aged 28-43 (mean, 36) years were instilled with sclerosant (povidone-iodine in three, ethanol in three, both povidone-iodine and ethanol in one). All seven patients subsequently experienced less abdominal pain. After drainage via an 8.5-Fr pigtail catheter inserted in the PICs (transabdominally in six cases, transvaginally in one), sclerosant equivalent in volume to about one-third that of drained fluid was introduced daily until the drained volume was less than 5ml. Follow-up by means of clinical procedures and ultrasound was performed every three months, at which time the success rate, possible complications and recurrence were determined.
Results
Sclerotherapy was technically successful in all seven patients, though immediately after the procedure, minor complications were noted in three patients (mild pain in two, mild fever in one). During the follow-up of 4-60 (mean, 24.7) months, sclerotherapy proved successful and without long-term complications in all seven patients: lower abdominal pain disappeared and the diameter of the cysts decreased more than 50%, with complete regression in four cases. During the follow-up period there was no recurrence.
Peritoneal inlcusion cysts (PICs) have been referred to variously as peritoneal cysts, inflammatory cysts of the pelvic peritoneum, benign cystic mesotheliomas, multilocular peritoneal cysts, ovarian pseudocysts, entrapped ovarian cysts, or a combination of these terms (1-4). Those that sometimes occur as a sequela of pelvic surgery, endometriosis or pelvic inflammatory disease consist of a collection of localized peritoneal fluid (1, 5-12).
Several published reports have described the successful radiologic diagnosis of PICs (1, 6, 7, 9, 13-17), and a number of these have suggested that they may be treated conservatively (1, 5-11, 15, 18). One such alternative to surgery is aspiration of the fluid collection in order to relieve the acute symptoms (1, 7-9); another is hormonal treatment using oral contraceptives (9, 18) or a gonadotropin-releasing hormone agonist (10). Sclerotherapy has recently been used effectively and successfully in the treatment of cysts of the kidney, liver, ovary and thyroid, and in the treatment of postoperative lymphocele (19-23), but to our knowledge, few reports have described the application of sclerotherapy to PICs (5).
The purpose of this study was to assess the technical feasibility and clinical effectiveness of catheter drainage and sclerotherapy for the treatment of PICs in a series of seven patients.
Between June 1996 and February 2001, seven female patients referred by gynecologists underwent sclerotherapy. The criteria for their selection were that they were symptomatic and showed the radiologic features of PICs. In all seven, sclerotherapy was indicated. All were premenopausal and aged between 28 and 43 (mean, 36) years. None were using oral contraceptives or other hormonal medications. All had a past medical history of pelvic surgery, as detailed in Table 1, having undergone total abdominal hysterectomy for uterine leiomyoma (n=3), cystectomy and adhesiolysis due to previous tuberculosis (n=2), leiomyomectomy of the uterus (n=1), or multiple operations for traumatic intestinal rupture or PIC (n=1). A diagnosis of PIC was established on the basis of clinical history and the imaging findings of ultrasound, CT, or MRI. The mean time interval between previous surgery and diagnosis was 5.7 (range, 1-13) years. Tumor markers such as CEA, AFP, CA125 and CA19-9 were checked at initial diagnosis, and informed consent for sclerotherapy was obtained from each patient.
We performed sclerotherapy in a total of eight PICs in seven patients. The greatest diameter of each cyst was 6-13 (mean, 9.3) cm, and they were located in the pelvic cavity (on the right side in two patients, on the left side in four, and on both sides in one), sometimes with lower abdominal extension. In the angiographic unit, we initially inserted an 8.5-Fr Ultrathane drainage catheter (Cook, Bloomington, Ind., U.S.A.) using a 0.035-inch-diameter Radiofocus M guide wire (Terumo, Tokyo, Japan) under the guidance of ultrasound and/or fluoroscopy. Insertion was transabdominal in seven cysts of six patients and transvaginal in the remaining one cyst of one patient in whom the transabdominal route was unsuitable due to overlying small intestine. Immediately after draining as much fluid as possible, a sclerosing agent was instilled and the drained fluid was sent for cytologic examination. The agent chosen was a 10% povidone-iodine topical solution (SungKwang Pharm, Bucheon, Korea) in three patients, absolute ethanol (J. T. Baker, Deventer, Holland) in three others, and both povidone-iodine and ethanol in one. The volume of sclerosing agent introduced was about one-third of that of the drained fluid [20-50 (mean, 41) ml]. After the sclerosant was injected into the cysts, patients were asked to change their positions in order to bring the entire wall of the cyst into contact with the sclerosant. The agent was drained naturally after 15 minutes in the case of ethanol and after one hour in the case of povidone-iodine. The amount, color and odor of newly drained fluid, as well as the position and patency of the catheters were checked daily in all patients prior to further instillation of the sclerosant. The volume of the subsequently introduced sclerosant was also about one-third of the volume of newly drained fluid. When less than 5ml was drained, the catheter was removed and sclerotherapy ended.
If these successive procedures were completed, the overall procedure was regarded as technically feasible; if a patient's symptoms improved and the diameter of a cyst decreased by more than 50%, the procedure was deemed clinically successful. In each patient, subsequent follow-up involved clinical and ultrasound examinations in the outpatient clinic at three-month intervals. We analyzed the technical feasibility of sclerotherapy, the immediate success rate, possible complications, and recurrence during the follow-up period.
The patients' primary diagnosis, previous surgical procedures, records concerning sclerotherapy of PICs, and outcomes, are summarized in Table 1. Sclerotherapy was technically successful and well tolerated in all patients and no major procedural complications occurred (Figs. 1, 2). As minor complications, mild abdominal pain in two patients (patients 1 and 5) and mild fever in one (patient 6) were noted immediately after the procedure and controlled adequately with analgesics. The aspirated fluid specimens were dark yellow (n=3), clear yellow (n=3), clear yellow (n=1), or dark and bloody (n=1). Cytologic examination revealed the presence of some mesothelial and inflammatory cells, but no evidence of granulomatous inflammation or malignancy, findings which are consistent with those of earlier reports (3, 7, 9, 12). In two patients with a history of tuberculosis (patients 3 and 7), there was no evidence of either this or malignancy in the drained fluid. The sclerosant was instilled in each patient 2-5 (mean, 3.1) times.
During the follow-up period of 4-60 (mean, 24.7) months, the symptoms of lower abdominal pain disappeared in all patients, and follow-up ultrasonography showed that the diameter of all cystic lesions decreased by more than 50%, disappearing completely in four patients (57.1%) Neither long-term complications nor recurrence were noted during the follow-up period.
Because cystic lesions in the pelvic cavity are common in postpubertal women (7), a cystic adnexal lesion prompts a long list of differential diagnoses, including ovarian cancer (5, 6). However, in premenopausal women with peritoneal adhesions due to previous surgery, endometriosis or pelvic inflammatory disease (2, 3, 6-8, 12, 18), PIC should be included in the differential diagnosis (1, 2, 6-8). It has been suggested, moreover, that these cysts are more common than had been thought (1, 4, 7, 15, 16, 24). In our study, a diagnosis of PIC was based on a patient's previous PIC-related clinical and radiologic characteristics (1, 7-9, 13, 14). Jain (1) asserted that if a lesion occurred outside the ovary, and this was normal and ipsilateral, definitive diagnosis was possible, and he summarized the imaging characteristics of PIC as follows: entrapped ovary in a spider web pattern, with peritoneal adhesions extending across the entire width of a fluid collection, unlike hydrosalpinx and pyosalpinx, and intact normal ipsilateral ovary. In addition, the values of tumor makers such as CEA, AFP, CA125 and CA19-9 were normal in our patients, and aspirated fluid cytology examination revealed no malignant cells. A PIC frequently becomes multiple, and large enough to be symptomatic (7). Pelvic pain or mass is usually a presenting feature (1-3, 6), and occurred in all seven of our patients. In addition, a PIC patient may be at risk of infertility, presumably due to scarring or the formation of adhesions around the adnexa (7). The treatment of symptomatic PIC is therefore essential, while for asymptomatic patients, simple observation may suffice (5, 7). Only when these cystic masses were shown by radiologic studies and laboratory data to be PICs, and the cause of pelvic pain, was sclerotherapy indicated.
Previously, PIC was treated mainly by surgical resection. Gussman et al. (8) believed that surgery was the treatment of choice and that percutaneous drainage was probably helpful. Although the treatment of choice for PIC has not been firmly established, the consensus is that conservative management is preferable to surgery (1, 5-11, 15, 18). The reasons for this are as follows: first, PICs are pathologically reactive, not neoplastic benign lesions (1-3, 6, 8, 10-12). Histopathologically, the locules are lined by one or several layers of flat, cuboidal mesothelial cells, which occasionally form papillae (1). Even when the cuboidal cells undergo squamous metaplasia, PICs have no malignant potential (1, 2). Second, because PICs adhere to the surface of the ovary but do not involve the ovarian parenchyma, and most patients are, moreover, premenopausal, oophorectomy is not recommended (3, 9). Third, after surgical resection, the recurrence rate is 30-50% (2). Fourth, PICs tend to rupture frequently just after the abdomen is opened, and resection is difficult because tissue planes are poorly defined (8). Fifth, because most patients are symptomatic and have previously undergone several laparotomies, additional surgical intervention is undesirable (1, 5).
Conservative alternatives to surgery include aspiration of the collected fluid to relieve the acute symptoms (1, 7-9), hormonal treatment using oral contraceptives (9, 18) and a gonadotropin-releasing hormone agonist (10). Oral contraceptives suppress ovulation, preventing accumulation by decreased fluid production (1, 7). Additionally, Kurachi et al (10) reported that after gonadotropin-releasing hormone agonist treatment, PICs became smaller. They also indicated, however, that the therapeutic value of these hormonal treatments might be limited in that their effect was short-lived and they failed to resolve the adhered lesions (10, 11).
Previous reports have suggested that aspiration is a safe and effective nonsurgical treatment for PICs (7, 8, 15, 17); simple aspiration is, however, associated with a high recurrence rate (1-3, 5, 14, 25, 26), as in the aspiration of simple renal cysts, hepatic cysts and postoperative lymphocele (19, 20, 23). We therefore believe that sclerotherapy following drainage is essential for the treatment of PICs. Kairuloma et al. (27) emphasized the need to bring the sclerosant into contact with the entire mucosal surface of the cyst, though since the majority of PICs have septations, this may not be easy (6, 9, 14). These septations are sometimes related to recurrence after sclerotherapy, and we therefore disrupted them by manipulating the guidewire during the procedure.
Our study suffers from certain limitations. First, the number of patients involved was quite small, and subsequent series should therefore involve more subjects. Second, because sclerotherapy was not performed in consecutive patients, but only in those who were referred, our evaluation of its effectiveness in treating PICs may be limited. Third, two different sclerosing agents were used, and we do not know which was better.
Because certain technical standards-such as those relating to the amount of sclerosant and the duration of sclerosant instillation-have not yet been established, the procedure may vary according to the authors involved (5). Our methods rely, basically, on the techniques used in percutaneous aspiration and sclerotherapy of lymphocele after surgery, or in the treatment of renal cysts. For initial sclerotherapy of PICs, we adopted the sclerotherapeutic method used for lymphocele because both PICs and lymphocele share in common the fact that they occur after pelvic surgery. At that time, povidone-iodine was used for lymphocele, but we replaced this with ethanol, which was effective in the sclerotherapy of simple renal cysts and might enhance the therapeutic effectiveness of sclerotherapy of PICs. Thus, we used two kinds of sclerosing agent: povidone-iodine and ethanol. Because our patients were few in number, however, we were unable to compare their effectiveness.
Although more extensive studies are needed, we believe that our successful results, with no recurrence, are because our approach involved intensive sclerotherapy until fluid drainage was minimal. In our study, no serious complications such as viscus perforation, infection, bleeding, or the spillage of cystic fluid and/or sclerosant into the peritoneal cavity occurred during or after sclerotherapy, and the results are promising.
In conclusion, these preliminary results indicate that sclerotherapy following catheter insertion appears to be technically feasible and effective for the treatment of PICs.
Figures and Tables
Fig. 1
A 37-year-old woman who underwent total abdominal hysterectomy for uterine leiomyoma ten years earlier presented with lower abdominal pain (Patient 1).
A. Enhanced CT shows an elongated cystic mass with no solid component on the left side of the pelvis (arrows).
B. Transabdominal ultrasonogram of the pelvis in the transverse plane indicates a persistent cystic mass with internal septation (arrow-heads) after simple aspiration. The left ovary may be observed (arrow).
C. An 8.5-Fr pigtail catheter was introduced into the lesion transabdominally, and povidone-iodine was used for sclerotherapy.
D. Transvaginal ultrasonogram obtained 54 months after the procedure shows scanty fluid (arrows) around the left ovary (LO).
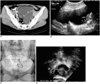
Fig. 2
A 28-year-old woman who had taken anti-tuberculosis medication for tuberculous peritonitis seven years earlier presented with lower abdominal pain (Patient 3).
A-C. T2-weighted (A), T1-weighted (B), and contrast-enhanced T1-weighted (C) MR images of the pelvis show a cystic mass 13cm in diameter. On the right side of the huge cystic lesion, the intact left ovary (arrow) is noted; the right ovary (curved arrow) is normal.
D. Transvaginal ultrasonogram reveals a cystic mass encircling the left ovary (arrow), which is displaced to the right of the pelvis. (This image was obtained transvaginally, and the ultrasonic probe is displayed at the top.)
E. An 8.5-Fr pigtail catheter was inserted transabdominally. Cytologic examination of the drained fluid, prior to sclerotherapy with absolute ethanol, revealed no evidence of tuberculosis or malignancy.
F. Transabdominal ultrasonogram in transverse plane obtained seven months later shows a 4 cm-sized cyst with progressive reduction in size (white arrow). The right ovary is also visible (open arrow).
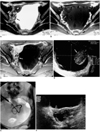
Table 1
Sclerotherapy Data for Seven Female PIC Patients

Note.-*PMHX= past medical history, TAH=total abdominal hysterectomy
**Sx= symptom, LAP= lower abdominal pain
†PIC= peritoneal inclusion cyst, Interval= time interval between previous surgery and the detection of PIC, N= negative
‡TA= transabdominal, TV= transvaginal, P= povidone-iodine, E= ethanol, Volume= volume of sclerosant introduced at initial sclerotherapy, Cx= complication, N= negative
¶Sx= symptom, Cx= complication, N= negative
References
1. Jain KA. Imaging of peritoneal inclusion cysts. AJR. 2000. 174:1559–1563.
2. Ross MJ, Welch WR, Scully RE. Multilocular peritoneal inclusion cysts (so-called cystic mesotheliomas). Cancer. 1989. 64:1336–1346.
3. McFadden DE, Clement PB. Peritoneal inclusion cysts with mural mesothelial proliferation: A clinicopathological analysis of six cases. Am J Surg Pathol. 1986. 10:844–854.
4. Schneider V, Partridge JR, Gutierrez F, Hurt WG, Maizels MS, Demay RM. Benign cystic mesothelioma involving the female genital tract: report of four cases. Am J Obstet Gynecol. 1983. 145:355–359.
5. Lipitz S, Seidman DS, Schiff E, Achiron R, Menczer J. Treatment of pelvic peritoneal cysts by drainage and ethanol instillation. Obstet Gynecol. 1995. 86:297–299.
6. Sohaey R, Gardner TL, Woodward PJ, Peterson CM. Sonographic diagnosis of peritoneal inclusion cysts. J Ultrasound Med. 1995. 14:913–917.
7. Hoffer FA, Kozakewich H, Colodny A, Goldstein DP. Peritoneal inclusion cysts: ovarian fluid in peritoneal adhesions. Radiology. 1988. 169:189–191.
8. Gussman D, Thickman D, Wheeler JE. Postoperative peritoneal cysts. Obstet Gynecol. 1986. 68(3):Suppl. 53S–55S.
9. Kim JS, Lee HJ, Woo SK, Lee TS. Peritoneal inclusion cysts and their relationship to the ovaries: evaluation with sonography. Radiology. 1997. 204:481–484.
10. Kurachi H, Murakami T, Maeda T, et al. Value of gonadotropin-releasing hormone agonist in diagnosing peritoneal pseudocysts. Acta Obstet Gynecol Scand. 1996. 75:294–297.
11. Takeuchi K, Kitazawa S, Kitagaki S, Mauro T. Conservative management of post-operative peritoneal cysts associated with endometriosis. Int J Gynecol Obstet. 1998. 60:151–154.
12. Rosai J. Rosai J. Peritoneum, retroperitoneum, and related structures. Ackerman's surgical pathology. 1996. St. Louis: Mosby;2137.
13. Cancelmo RP. Sonographic demonstration of multilocular peritoneal inclusion cyst. J Clin Ultrasound. 1983. 11:334–335.
14. Lees RF, Feldman PS, Brenbridge AN, Anderson WA, Buschi AJ. Inflammatory cysts of the pelvic periteum. AJR. 1978. 131:633–636.
15. Kurachi H, Murakami T, Nakamura H, et al. Imaging of peritoneal psuedocysts: value of MR imaging compared with sonography and CT. AJR. 1993. 160:589–591.
16. Hederstrom E, Forsberg L. Entrapped ovarian cyst-an unusual case of persistent abdominal pain. Acta Radiol. 1990. 31:285–286.
17. Fleischer AC, Tait D, Mayo J, Burnett L, Simpson J. Sonographic features of ovarian remnants. J Ultrasound Med. 1998. 17:551–555.
18. Komickx PR, Renaer M, Brosens IA. Origin of peritoneal fluid in women: an ovarian exudation product. Br J Obstet Gynaecol. 1980. 87:177–183.
19. Hanna RM, Dahniya MH. Aspiration and sclerotherapy of symptomatic simple renal cysts: value of two injections of a sclerosing agent. AJR. 1996. 167:781–783.
20. Simmonetti G, Profili S, Sergiacomi GL, Meloni GB, Orlacchio A. Percutaneous treatment of hepatic cysts by aspiration and sclerotherapy. Cardiovasc Intervent Radiol. 1993. 16:81–84.
21. Troiano RN, Taylor KJ. Sonographically guided therapeutic aspiration of benign-appearing ovarian cysts and endometriomas. AJR. 1998. 171:1601–1605.
22. Cho YS, Lee HK, Ahn IM, et al. Sonographically guided ethanol sclerotherapy for benign thyroid cysts: results in 22 patients. AJR. 2000. 174:213–216.
23. Zuckerman DA, Yeager TD. Percutaneous ethanol sclerotherapy of postoperative lymphoceles. AJR. 1997. 169:433–437.
24. Philip G, Reilly AL. Benign cystic mesothelioma. Br J Obstet Gynaecol. 1984. 91:932–938.
25. Katsube Y, Mukai K, Silverberg SG. Cystic mesothelioma of the peritoneum: a report of five cases and a review of the literature. Cancer. 1982. 50:1615–1622.
26. Miles JM, Hart WR, McMahon JT. Cystic mesothelioma of the peritoneum. Report of a case with multiple recurrences and a review of the literature. Cleve Clin Q. 1986. 53:109–114.
27. Kairuloma M, Leinonen A, Stanlberg M, Paivansalo M, Kiviniemi H, Siniluoto T. Percutaneous aspiration and sclerotherapy for symptomatic hepatic cysts. Ann Surg. 1989. 210:208–215.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download