Abstract
Objective
The purpose of this study is to correlate the findings of peripheral hypoechoic rim, seen at transrectal ultrasonography (TRUS) in chronic prostatitis patients, with the histopthologic findings.
Materials and Methods
Seven patients with pathologically proven chronic prostatitis were involved in this study. The conspicuity of the peripheral hypoechoic prostatic rim, seen at TRUS, was prominent and subtle, and to determine its histopathologic nature, the microscopic findings were reviewed.
Results
In five of seven cases (71%), TRUS demonstrated a prominent peripheral hypoechoic rim. Microscopic examination revealed that inflammatory cell infiltration of prostatic glandular tissue was severe in three cases (42.9%), moderate in two (28.6%), and minimal in two (28.6%). In all seven cases, the common histopathologic findings of peripheral hypoechoic rim on TRUS were loose stromal tissues, few prostatic glands, and sparse infiltration by inflammatory cells.
In recent years, transrectal ultrasound (TRUS) examination of the prostate has come to be recognized as a valuable technique for the differential diagnosis of the prostatic disease (1). Although established criteria help to diagnose benign hyperplasia and carcinoma, little attention has been paid to the role of TRUS in the diagnosis of prostatitis (2).
A peripheral hypoechoic halo around the central zone of the prostate, seen at TRUS, is a common finding in benign prostatic hyperplasia, of which the histopathologic findings have been well established. A thin hypoechoic halo surrounds the hyperplastic nodule and this surgical capsule is composed of connective tissue or the compressed outer zone of the prostate (3-5). In our experience, a hypoechoic rim occupying the ourter periphery of the prostate is also frequently encountered at TRUS in patients with suspected chronic prostatitis. The histopathologic characteristics of this finding are not, however, well known.
The purpose of our study was to assess the histopathologic findings of hypoechoic rim at the outer periphery of the prostate, as seen at TRUS in cases of prostatitis.
Between January 1995 and July 1998, chronic prostatitis was diagnosed in 62 patients who underwent TRUS. Heterogenous echo patterns or increased high-density echoes of the prostate were observed. Because of abnormalities discovered at digital rectal examination, or elevated prostate-specific antigen, 14 of the 62 underwent systematic sextant core biopsy, and infiltration by inflammatory cells was confirmed. Although the microscopic findings suggested inflammatory cell infiltration, seven patients whose biopsy specimens indicated poor anatomical orientation of the central and peripheral portions of the prostate were excluded because of the difficulty of histopathologic correlation with the peripheral hypoechoic rim. This anatomical orientation was established by confirming the presence of a periprostatic venous plexus or neurovascular bundles located exclusively in periprostatic tissues. Finally, seven patients aged 52 to 69 (mean 63) years in whom infiltration by inflammatory cells was observed, with good orientation of the central and peripheral portions, were included in our study.
All sonographic examinations were performed by three of the authors (H.J.L., C.G.S., S.H.K.), using a 7-MHz end-firing endoluminal transducer with an HDI unit (Advanced Technology Laboratories, Bothell, Wash., U.S.A.). For ultrasonography and biopsies, all patients were in the left lateral decubitus position. All underwent gray-scale TRUS of the entire prostate and seminal vesicles in the sagittal and transverse planes.
TRUS enabled us to grade as 'prominent' or 'subtle' the conspicuity of the hypoechoic rim at the outer periphery of the prostate. If a rim over 3 mm thick was seen to extend to the lateral end of the prostate without evidence of disruption, conspicuity was graded as 'prominent' (Fig. 1A), but if the rim was obscure to that point, the grade assigned was 'subtle' (Fig. 2A).
After gray-scale TRUS, systematic sextant core biopsies at six sites in the upper, mid, and lower portions of the prostate were performed transrectally using an automatic biopsy gun (Pro-Mag 2.2, Manan Medical Products, Northbrook, Ill., U.S.A.) mounted with an 18-gauge needle of 17-mm core length. In order to obtain satisfactory specimens which included the hypoechoic rim of the outer periphery of the prostate seen at TRUS, the needle tip was located outside the prostate.
One pathologist (G.Y.C.) analyzed the biopsy specimens without prior knowledge of the TRUS findings. Cases were classified as severe, moderate, or minimal, according to the degree of inflammation in prostate glandular tissues. The 'severe' group included cases in which destruction of prostate glandular tissue due to inflammatory cell infiltration was observed, while the 'moderate' classification was assigned to cases showing massive infiltration by inflammatory cells without evidence of prostate glandular tissue destruction. Those in which there was little infiltration by inflammatory cells were classified as 'minimal'. The histopathologic findings obtained were correlated with those of TRUS.
Table 1 summarizes the results of TRUS and the pathologic findings. Among seven cases, TRUS indicated that the hypoechoic rim at the outer periphery of the prostate was prominent in five (71.4%) and subtle in two (28.6). Pathologically, inflammatory cell infiltration of prostate glandular tissue was severe in three cases (42.9%), moderate in two (28.6%), and minimal in two (28.6%).
In all seven cases, the microscopic findings of the peripheral prostatic band, thought to correspond to the peripheral hypoechoic rim seen at TRUS, were loose stromal tissues, few prostatic glands, and sparse infiltration by inflammatory cells (Figs. 1, 2). The other portion, thought to correspond to the heterogeneous echogenic area demonstrated by TRUS, showed varying degrees of inflammatory cell infiltration of prostatic glandular tissue.
Prostatitis is the most common urologic disease in men aged less than 50, and the third most common in men over 50 (6). A recent population-based study of one community suggested a prevalence of 9% (7). Dominant symptoms of pain, frequency, urgency, and nocturia overlap with those of other lower urinary tract disorders. The findings of digital rectal examination in patients with chronic prostatitis vary from normal, boggy or tender to indurations or nodularity (7).
In the peripheral acinar zone, neoplasia and inflammation predominate. Specifically, prostate cancer selectively originates in peripheral acinar tissue (8); prostatitis also originates selectively in the peripheral zone, as does cancer (9,10). Chronic inflammation may lead to atrophy of the gland at a late stage (8).
Ultrasonography is an important tool in the routine evaluation of patients in whom prostatic disease is suspected. Not only does it provide important information with respect to the presence of underlying disease, but is used to determine the most suitable route for prostatic biopsy (11); accurate and ultrasonographically controlled placement of the biopsy needle enables histologic assessment of the parenchymal features. Microscopic examination for chronic prostatitis reveals collections of lymphocytes, plasma cells, and a few macrophages. Infiltration by inflammatory cells may be insufficient to warrant mention in the pathology report, or may be sufficiently extensive to obliterate the normal architecture (7). In our study, prostatic inflammation was classified as to severe, moderate or minimal, according to the extent of inflammatory cell infiltration and destruction of normal prostate gland.
The reports about TRUS findings in chronic prostatitis vary (2). According to Doble and Carter, the seven features revealed by ultrasound were high-density echoes, mid-range echoes, echo-lucent zones, ejaculatory duct calcification, capsular irregularity, capsular thickening, and periurethral zone irregularity (2). In a study of whole-organ sections of 50 prostate glands obtained at either radical prostatectomy for adenocarcinoma or cystoprostatectomy for bladder cancer, Ayala et al. (12) found that the prostatic capsule consists of a band of concentrically placed fibromuscular tissue that is an inseparable component of prostatic stroma. The outer surface of this tissue consists of a few bundles of fibromuscular stroma that penetrate and disappear into the periprostatic connective tissue stroma. Lower power examination reveals that most peripheral glandular units do not reach the outer margin of the prostate and there is a 2- to 3-mm band of stromal tissue separating glandular units from periprostatic connective tissue (12). These findings are consistent with ours describing the hypoechoic rim found in chronic prostatic inflammation. In our study, microscopy revealed that in the peripheral prostatic zone, glandular elements in the stromal tissue band and inflammatory cell infiltration around the glandular tissue were both sparse. Increased or heterogeneous echo in the central portion of the prostate correlated with inflammatory cell infiltration of its glandular tissues, and the appearance of its peripheral hypoechoic rim-composed mainly of fibromuscular stoma and a few glandular tissues-indicated sparse infiltration by inflammatory cells.
Our study suffers from certain limitations. Because it focused on the histopathology of the peripheral hypoechoic rim revealed by TRUS, many cases in which this rim was present were excluded because no biopsy was performed. Even though our study did not predict sensitivity or specificity in the diagnosis of chronic prostatitis, we were able to correlate the histopathologic findings with those of TRUS.
In conclusion, the hypoechoic rim observed at TRUS at the outer periphery of the prostate in patients with prostatic inflammation reflects a sparsity of prostatic glandular tissue and is thought to be an area in which inflammatory cell infiltration is minimal. The presence of a peripheral hypoechoic prostatic rim may therefore help in the diagnosis of prostatic inflammation.
Figures and Tables
Fig. 1
A 64-year-old man with prominent hypoechoic rim seen at Transrectal ultrasonography (TRUS) and severe inflammatory cell infiltration.
A. TRUS shows a hypoechoic rim extending to the lateral portion of the prostate (arrows).
B. At lower power field, microscopic image shows that the peripheral portion, seen at TRUS as a hypoechoic rim, is composed of loose connective tissue (arrows) (×40).
C. The peripheral portion of the gland is composed of fibrous tissue and smooth muscles (×200). Note the sparse infiltration by inflammatory cells.
D. Microscopic image of the central portion shows marked infiltration by inflammatory cells (×200).
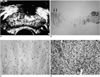
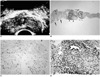
Fig. 2
A 60-year-old man with subtle hypoechoic rim and moderate inflammatory cell infiltration.
A. The hypoechoic rim seen at transrectal ultrasonography (TRUS) is less prominent than in Fig 1 (arrows).
B. Microscopic image shows loose peripheral connective tissue (arrows) and central glandular tissue with infiltrative inflammatory cells (arrowheads) (×40).
C. Image of the peripheral portion reveals sparse infiltration of fibrous tissue and smooth muscle by inflammatory cells (×200).
D. Image of the central portion shows that infiltration by inflammatory cells (×200) is less prominent than in Fig. 1.
References
1. Griffiths GJ, Crooks AJR, Roberts EE, et al. Ultrasonic appearances associated with prostatic inflammation: a preliminary study. Clin Radiol. 1984. 35:343–345.
2. Doble A, Carter S. Ultrasonographic findings in prostatitis. Urol Clin North Am. 1989. 16:763–770.
3. Burks DD, Drolshagen LF, Fleischer AC, Liddelle HT, McDougal WS, Karl EM, James AE Jr. Transrectal sonography of benign and malignant prostatic lesions. AJR. 1986. 146:1187–1191.
4. Langer JE. The current role of transrectal ultrasonography in the evaluation of prostatic carcinoma. Semin Roentgenol. 1999. 4:284–294.
5. Walsh PC. Walsh PC, editor. Benign prostatic hyperplasia. Campbell's Urology. 1992. 6th ed. Philadelphia: Saunders;1009–1027.
6. Collins MM, Stafford RS, O'lwary MP. How common is prostatitis? A national survey of physician visits. J Urol. 1998. 159:1224–1228.
7. Wasserman NF. Prostatitis: Clinical presentations and transrectal ultrasound findings. Semin Roentgenol. 1999. 4:325–330.
8. Rifkin MD. Endorectal sonography of the prostate: Clinical implications. AJR. 1987. 148:1137–1142.
9. McNeal JE. Origin and development of carcinoma in the prostate. Cancer. 1969. 23:24–31.
10. McNeal JE. Normal and pathologic anatomy of the prostate. Urology. 1969. 17:11–17.
11. Grossfeld GD, Coakley FV. Benign prostatic hyperplasia: Clinical overview and value of diagnostic imaging. Radiol Clin North Am. 2000. 38:31–47.
12. Ayala AG, Ro JY, Babaian R, Troncoso P, Grignon DJ. The prostatic capsule: Does it exist? Am J Surg Pathol. 1989. 13:21–27.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download