Abstract
Objective
To investigate the effectiveness of the newly designed Niti-S stent in the management of iliac arterial stenoses and occlusions.
Materials and Methods
Stenoses (n=25) or occlusions (n=5) in the iliac arteries of 25 patients (30 limbs) were treated. The site of the lesions was the common (n=15) or external (n=11) iliac artery, or both (n=4). Eight limbs were treated for diffuse disease, six for highly eccentric lesion, five for occlusive lesion, and 11 for failed angioplasty.
Results
In all patients, technical success was achieved without major complications. One death, not procedure-related, occurred within 30 days. Ankle-brachial indexes improved from 0.63±0.30 to 0.99±0.21, and ischemic symptoms showed improvement in 22 patients (88%). Fontaine classifications before stenting, namely IIa(n=3), IIb(n=16), III(n=2), and IV(n=4) improved to I(n=17), IIa(n=5), and IV(n=3). Follow-up over a 27 (mean, 19.8±8)-month period showed that cumulative patency rates were 95.8% at 1 year and 86.2% at 2 and 3 years. No significant decrease in the mean ankle-brachial index was observed.
Metallic stent placement has been widely accepted as safe and effective for the treatment of iliac arterial stenoses or occlusions (1-4). The superiority of such procedures compared with percutaneous transluminal angioplasty (PTA) for the treatment of iliac arterial occlusive disease has been described: meta-analysis of the results of both techniques has shown that the former has a higher technical success rate, together with less risk of long-term failure (5).
Nitinol is an alloy of nickel and titanium, and a good material for use in a self-expanding stent. It is flexible and suitable for transluminal placement below its transit temperature, and above this temperature resumes its original annealed shape. Since the report published by Dotter et al. in 1983 (6), several other reports have described clinical and experimental application of the nitinol stent (7, 8). The Strecker, Memotherm and Cragg stents are examples of nitinol stents which have recently become available (9-11). The advantages of such stents for intravascular applications are easy delivery, a high expansion rate, low metal to tissue ratio, and the lack of severe neointimal proliferation (12-14).
We recently designed and developed a nitinol, mesh-type, vascular stent interlaced with a nitinol monofilament. For the evaluation of in-vivo feasibility, an experiment involving dogs was performed (15), and to investigate the stent's clinical effectiveness, a clinical trial involving the management of iliac arterial stenoses and occlusions was conducted.
Under a protocol approved by an institutional review board, a Niti-S stent (Taewoong, Seoul, Korea) was placed in the iliac arteries of the 25 patients (30 limbs) between 10 March, 1998 and 4 August, 1999. Informed consent was obtained from the 24 men and one woman involved, whose mean age was 66.8 (range, 54-83) years. Coincident disease that may have predisposed the patients to vascular disease included cigarette smoking [n=21 (84%)], arterial hypertension [n=14(56%)], elevated serum cholesterol [n=11(44%)], and diabetes mellitus [n=8(32%)]. More than one coincident disease was present in 16 patients (64%). On the basis of the Fontaine classification (16-17), the clinical symptoms included three patients (12%) with mild claudication (Fontaine stage IIa), 16 (64%) with severe claudication (IIb), two (8%) with rest pain (III), and four (16%) with tissue loss or gangrene (IV). At patient presentation, vascular disease involved the common iliac arteries in 15 limbs, the external iliac arteries in 11, and both arteries in four. Lesions took the form of stenosis of over 75% (n=25) or complete occlusion (n=5), and their length varied from 2.0 to 10.0 (mean, 4.1) cm. Arterial runoff was as follows: the ipsilateral superficial femoral arteries were occluded in eight limbs, there was stenosis of over 75% in five, and the profunda femoris artery was occluded in one.
Ankle-brachial indexes (ABI) and intravascular pressures were measured before and after the procedure. Primary stent placement was performed in cases involving diffuse long segmental stenoses (greater than 5 cm; eight limbs), highly eccentric lesions (those at one side of a vessel wall; six limbs), or chronic occlusion (five limbs). In the remaining 11 limbs, secondary stent placement was performed after failed PTA. The indication for stent placement was depiction of a residual stenosis of at least 30% at anteroposterior and oblique angiography after PTA. Residual stenoses were a result of dissection or elastic recoil in five and six limbs, respectively.
The Niti-S stent was used in all patients. Its endoprosthesis consisted of an annealed 0.08-inch monofilament nitinol wire wound on the mandrel, thus creating a spiral mesh (Fig. 1). At room temperature, a nitinol stent softens and can be folded to a diameter of 2.5 mm without destroying its memory. At deployment, the stent self-expands to a predetermined diameter of 5-10 mm, with a length of 3-10 cm. Its mechanical properties and biocompatibility have been previously described in detail (15).
The ipsilateral femoral artery was punctured and a 7-Fr long sheath (Cook, Bloomington, Ind., U.S.A.) with dilator was located under fluoroscopic guidance. A 0.038-inch, angled-tip, hydrophilic guide wire (Terumo, Tokyo, Japan) with or without a 6-Fr angiographic catheter (Cook) was used in the initial attempt to across the lesion. If this failed, another attempt (the antegrade approach) was made from the contralateral common femoral artery, using the "modified wire-loop" technique (18). After crossing the lesion with the guide wire, the long sheath with dilator was advanced. The deployment technique for the Niti-S is not complicated. In order to load the stent into the long sheath, the former was first loaded into a 7-Fr short sheath, the distal end of which was inserted into the valve at the proximal end of the long sheath, which had already been placed at the desired site. The stent was delivered to the end of the long sheath using a pusher catheter, and once in its proper position, withdrawal of the long sheath permitted its deployment and expansion. To achieve full expansion, PTA after deployment was necessary in all patients. To exclude the possibility of iatrogenic distal embolization, postprocedural angiography was performed. Prior to stent deployment, a 3000-IU heparin bolus was administrated; for procedures lasting over two hours, infusions were repeated. One 325-mg aspirin tablet was prescribed daily, beginning the night before the procedure, and was continued for three months. Thrombolysis or atherectomy was not performed.
The clinical symptoms before and after treatment were determined on the basis of the Fontaine classification. Technical success was defined as exact deployment of the stent, less than 25% residual stenosis, and restoration of rapid, antegrade blood flow through the stent. Clinical success was defined as symptom improvement of at least one Fontaine stage.
Patients were followed up clinically at 1, 3, and 6 months after the procedure, and every 6 months thereafter. At these intervals, a history was taken, ABIs were measured, and color and duplex Doppler analyses of the stent were performed. Follow-up digital subtraction angiography was undertaken when clinical symptoms indicated the need for this, or if ABI had deteriorated during the follow-up period. Occlusion or stenosis of more than 50% was seen as restenosis, and for statistical analysis of the patency rates, Kaplan-Meier survival analysis was used.
Stents were successfully implanted in all 30 limbs, a single Niti-S stent being placed in each limbs; with no additional stent required (Fig. 2). In one patient with long segmental stenosis, the anterolateral central portion of a 6-cm-long stent was compressed due to strong arterial wall recoil. Repeated balloon dilation led to successful expansion, however. The mean pressure gradient across the lesion decreased significantly, from 50.2±31.1 to 3.8±1.3 mmHg. Residual stenosis of less than 25% remained in five limbs (16.6%), but the residual pressure was minimal [range, 0-5 (mean, 3) mmHg]. Mean ABI increased from 0.63±0.3 before the procedure to 0.99±0.21 after, a difference which according to the paired t test was statistically significant (p<0.01). The clinical symptoms improved by at least one Fontaine stage in 22 patients (88%), none of whom showed deterioration in their clinical stage. Three days after stenting, Fontaine stage I was observed in 17 patients, IIa in five, and IV in three. The symptoms of three Fontaine stage IV patients had not improved. Immediate angiographic follow-up indicated that the internal iliac artery was covered by the stent in 13 limbs, though patency was maintained in all limbs with the exception of one with significant stenosis. During the follow-up period, however, this patient showed no clinical symptoms or signs.
During follow-up of up to 27 (mean, 19.8±8) months, during which time two patients were lost, the clinical stage and ABI were well maintained. After 6, 12, and 24 months, ABIs were 0.99±0.09, 0.91±0.15, and 0.92±0.1, respectively. No patient showed a deterioration in clinical stage or an ABI decline of greater than 0.15. In two patients, restenosis was detected by follow-up Doppler sonography at 3 and 21 months, respectively. In one of these, intra-stent stenosis (75% of nominal diameter) was confirmed by angiography and successfully treated with PTA. The other patient declined further treatment. In addition to five limbs in which residual stenoses remained after stent placement, a newly developed stenosis (less than 25%) in the distal portion of the stent was revealed by follow-up sonography in two patients (two limbs). The modality showed that the condition of both subsequently remained stable, and further treatment was not attempted. Between two and 18 (mean, four) months later, despite a normally patent stent, bypass surgery was performed in four patients (five limbs) because other foci of atherosclerosis were seen distally or contralaterally. The cumulative patency rates were 95.8% at 1 year and 86.2% at 2 and 3 years (standard error, 10%).
No major procedure-related complications occurred. A puncture site pseudoaneurysm was observed in one patient, though this was successfully obliterated by sonoguided compression. One patient died of acute myocardial infarction within one month of the procedure, and during the follow-up period a further three died of diabetic nephropathy (n=2) or rupture of an abdominal aortic aneurysm (n=1).
Thirty complex lesions of the iliac artery were treated with this newly designed nitinol monofilament stent (Niti-S). Exact stent placement was possible in all patients and the initial technical success rate was 100%. In our study, the Niti-S stent showed several desirable features that made it well-suited for the treatment of iliac stenoses and occlusions. The 10-mm-diameter stent could be loaded into a 7-Fr sheath, and is thus quite compressible. It possesses good flexibility during both the implanted and sheath-loaded stage, allowing stenting through curved arteries over the hip joint. In treated arteries, its self-expanding force was sufficient to maintain an adequate diameter and, in addition, the radiopaque markers present at both ends facilitate deployment at the exact site. During deployment, however, marked shortening, similar to that exhibited by the Wallstent, was observed, and this made it somewhat difficult to accurately place the stent and imposed a risk of insufficient coverage of the diseased segment due to further expansion and shortening during follow-up. The stent is available in several lengths and diameters; it is, therefore, important to measure the lesion accurately, thus ensuring that the stent chosen is optimal.
In 88% of patients, symptoms of ischemia improved by at least one Fontaine stage, and in 64%, improvement of at least two grades was noted. A favorable result was obtained in stage-II patients: in twelve of the 16 who were stage IIb and all three who were IIa, clinical symptoms improved to stage I (no symptoms). In stage IV patients (n=4), the results were poor, however; the symptoms of three of the four did not improve even after successful stent placement, and they suffered uncontrolled diabetes and gangrenous change in their feet. Digital subtraction angiography showed multifocal stenoses or occlusions in all three trifurcating branches of the popliteal artery, and this was probably the cause of their symptoms. Eventually, the affected feet or toes were amputated. We believe that if stents had not already been deployed, more extensive surgery would probably have been possible. Uncontrolled diabetes appears, therefore, to be a contraindication for stent insertion.
At present, stent patency is limited mainly by early thrombosis and delayed restenosis. Early stent thrombosis may be related to technical problems and stent thrombogenicity, and delayed restenosis or occlusion can occur due to intimal hyperplasia (19, 20). In our study, early stent thrombosis (within one month) was not observed. In two patients, however, restenosis due to intimal hyperplasia developed three and 21 months, respectively, after stent placement. In the patient in whom restenosis (50% of nominal diameter) was detected by follow-up sonography 21 months after stent placement, no ischemic symptoms were observed and further evaluation was declined. During a follow-up period of 27 months, the degree of restenosis in one of these two patients was unchanged and he was free of symptoms. In the other, Doppler ultrasound and digital subtraction angiography performed three months after stent placement revealed severe (75%) intrastent stenosis of the distal portion, though this was successfully treated by PTA. In those two patients, angiography showed that the superficial femoral arteries were occluded. Poor runoff has been noted to adversely affect long-term patency rates in aortofemoral bypass grafts, in iliac artery angioplasty, and in stented iliac artery (21, 22). With regard to animal experiments in which neointimal hyperplasia lasted for up to six months, there was no significant difference between different stents, including the Palmaz, Wallstent and nitinol (Memotherm) types (19). Three to four weeks after insertion of the nitinol stent, the neointima was well organized, with an endothelial lining, subintimal fibrosis, and mild infiltration by inflammatory cells. In an experimental study in which a Niti-S stent was placed in canine iliac arteries, the neointima was thickest eight weeks after stent placement and was progressively replaced by collagen. Compared with previous reports, the neointimal thickness of the Niti-S stent was acceptable (17).
In the five patients with complete iliac arterial occlusion, the hemodynamic and clinical outcomes were not significantly different from those of arterial stenoses in the other 20. In those with complete occlusion, the mean systolic pressure gradient across the lesion improved from 54 to 4 mmHg. The clinical stage of these patients [IIb (n=4) and III (n=1)] improved to I. During the follow-up period, patency of the stented segment was well maintained. The mean ABI in the five patients was 0.84 before the procedure, 1.04 three days after its completion, and 1.02 at the end of the follow-up period, suggesting good clinical and hemodynamic outcomes.
The overall complication rate for stent placement has been reported as 6.7-23.7% (23, 24). In our study, with the exception of one pseudoaneurysm which was easily treated by compression, procedure-related complications were not observed. Though not a major drawback in primary stent placement, the distal embolization rate has been reported as 0-17% (25, 26).
In our study, close contact between the stent-loaded sheath and the lesion during the procedure seems to have prevented embolization. In most interventions, distal embolization is prevented though the routine use of antiplatelet agents. The most common of these is aspirin, though several others, such as dipyridamole and ticlopidine, have also been used. Controlled clinical studies have, however, failed to demonstrate the antithrombotic efficacy of dipyridamole where this is used alone (27); ticlopidine, on the other hand, has proven superior to aspirin for the prevention of thromboembolism (28).
In conclusion, the technical success and complication rates for percutaneous iliac artery revascularization using the Niti-S stent were favorable, the symptoms of most patients improved, and patency rates were comparable to those obtained using other commercially available stents. We believe, though, that it is necessary to determine the long-term effectiveness of this stent, and for this, a larger series of patients should be involved.
Figures and Tables
 | Fig. 1The Niti-S consists of a monofilament nitinol wire wound on the mandrel in such a way that a series of interlacing wires creates a spiral mesh around its circumference. The distance between bends is approximately 2mm, and both ends of the wire are connected at the central portion of the stent (arrow). The leading and trailing ends of the stent are coated with platinum, which helps identify them and facilitates deployment (curved arrows). |
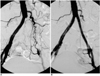 | Fig. 2
A. Pelvic angiogram obtained in a 68-year-old insulin dependent diabetic man with left-sided Fontaine stage-IIb claudication shows segmental occlusion of about 8cm involving the left common iliac artery, with opacification of the external iliac artery and numerous collateral channels. The translesional mean pressure gradient was 50 mmHg.
B. Angiogram obtained after primary stent placement shows good patency of a previously occluded segment, and there is no residual pressure gradient across the lesion. During follow-up lasting 23 months, the patient was free of symptoms.
|
References
1. Murphy KD, Encarnacion CE, Le VA, Palmaz JC. Iliac artery stent placement with the Palmaz stent; follow-up study. J Vasc Interv Radiol. 1995. 6:321–329.
2. Liermann D, Strecker EP, Peters J. The Strecker stent: indications and results in iliac and femoropopliteal arteries. Cardiovasc Intervent Radiol. 1992. 1:298–305.
3. Murphy TP, Webb MS, Lambiase RE, et al. Percutaneous revascularization of complex iliac artery stenoses and occlusions with use of Wallstents: three-year experience. J Vasc Interv Radiol. 1996. 7:21–27.
4. Yedlich JW, Ferral H, Bjarnason H, Hunter DW, Castaneda-Zuniga WR, Amplatz K. Chronic iliac artery occlusion: primary recanalization with endovascular stents. J Vasc Interv Radiol. 1994. 5:843–847.
5. Bosch JL, Hunink MGM. Meta-analysis of the results of percutaneous transluminal angioplasty and stent placement for aortoiliac occlusive disease. Radiology. 1997. 204:87–96.
6. Dotter CT, Buschmann RW, McKinney MK, Rosch J. Transluminal expandable nitinol coil stent grafting: preliminary report. Radiology. 1983. 147:259–260.
7. Cragg AH, Dejong S, Barnhart W, Landas S, Smith TP. Preliminary evaluation of the Cragg stent (abstr). Radiology. 1992. 185(P):162.
8. Cragg AH, Lund G, Rysavy JA, Salomonowitz E, Castaneda-Zuniga WR, Amplatz K. Percutaneous arterial grafting. Radiology. 1984. 150:45–49.
9. Foelich JJ, Alfke H, Wilke A, et al. Effects of nitinol Strecker stent placement on vascular response in normal and stenotic porcine iliac arteries. J Vasc Interv Radiol. 1999. 10:329–338.
10. Shaw JW. Management of aortoiliac occlusive vascular disease with the Memotherm self-expanding nitinol stent. J Intervent Radiol. 1996. 11:119–127.
11. Maynar M, Reyes R, Ferral H, et al. Cragg endopro system I: early experience. I. Femoral arteries. J Vasc Interv Radiol. 1997. 8:203–207.
12. Hausegger KA, Cragg AH, Lammer J, et al. Iliac artery stent placement: clinical experience with a nitinol stent. Radiology. 1994. 190:199–202.
13. Wakloo AK, Tio FO, Lieber BB, et al. Self-expandable nitinol stents in canine vertebral arteries: hemodynamics and tissue response. Am J Neuroradiol. 1995. 16:1043–1051.
14. Schwarzenberg H, Muller-Hulsbeck S, Gluer CC, Steffens JC, Heller M. Evaluation of maximum neointima proliferation and plaque morphology in iliac self-expanding nitinol stents with intravascular sonography. AJR. 1998. 171:1627–1630.
15. Lee KW, Park JH, Chung JW, Kim WS, Lee W, Yeon KM. Short-term effects of a new intravascular nitinol stent in canine arteries. Invest Radiol. 1999. 34:367–373.
16. Pentecost MJ, Criqui MH, Dorros G, et al. Guidelines for peripheral percutaneous transluminal angioplasty of the abdominal aorta and lower extremity vessels. Circulation. 1994. 89:511–531.
17. Rutherford RB, Necker GJ. Standards for evaluating and reporting the results of surgical and percutaneous therapy for peripheral arterial disease. J Vasc Interv Radiol. 1991. 2:169.
18. McLean GK, Cekirge S, Weiss JP, Foster RG. Stent placement for iliac artery occlusions: modified "wire-loop" technique with use of the goose-neck loop snare. J Vasc Interv Radiol. 1994. 5:701–703.
19. Palmaz JC, Laborde JC, Rivera FJ, et al. Stenting of the iliac arteries with the Palmaz stent: experience from a multicenter trial. Cardiovasc Intervent Radiol. 1992. 15:291–297.
20. Schurmann K, Vorwerk D, Kulisch A, et al. Neointimal hyperplasia in low-profile nitinol stents, Palmaz stents, and Wallstents: a comparative experimental study. Cardiovasc Intervent Radiol. 1996. 19:248–254.
21. Ballard JL, Sparks SR, Taylor FC, et al. Complications of iliac artery stent deployment. J Vasc Surg. 1996. 24:545–555.
22. Blum U, Gabelmann A, Redecker M, et al. Percutaneous recanalization of iliac artery occlusions: results of a prospective study. Radiology. 1993. 189:536–540.
23. Laborde JC, Dougherty S, Rivera FJ, Encarnacion CE, Palmaz JC. Influence of anatomic distribution of atherosclerosis in the outcome of iliac revascularization (abstract). J Vasc Interv Radiol. 1992. 3:31.
24. Stokes KR, Strunk HM, Campbell DR, Gibbons GW, Wheeler HG, Clouse ME. Five-year result in iliac and femoropopliteal angioplasty in diabetic patients. Radiology. 1990. 174:977–982.
25. Gunther RW, Vorwerk D, Bohndorf K, Peters I, el-Din A, Messmer B. Iliac and femoral artery stenoses and occlusions: treatment with intravascular stents. Radiology. 1989. 172:725–730.
26. Long AL, Page PE, Raynaud AC, et al. Percutaneous iliac artery stent: angiographic long-term follow-up. Radiology. 1991. 180:771–778.
27. Fitzgerald GA. Dipyridamole. N Engl J Med. 1987. 316:1247–1257.
28. Montalescot G. Value of antiplatelet therapy in preventing thrombotic events in generalized vascular disease. Clin Cardiol. 2000. 23:18–22.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download