Abstract
Objective
To retrospectively evaluate the impact of pedal arch quality on tissue loss and time to healing in diabetic patients with foot wounds undergoing infrainguinal endovascular revascularization.
Materials and Methods
Between January 2014 and June 2015, 137 consecutive diabetic patients with foot wounds underwent infrainguinal endovascular revascularization (femoro-popliteal or below-the-knee, arteries). Postprocedural angiography of the foot was used to divide the patients into the following three groups according to the pedal arch status: complete pedal arch (CPA), incomplete pedal arch (IPA), and absent pedal arch (APA). Time to healing and estimated 1-year outcomes in terms of freedom from minor amputation, limb salvage, and survival were evaluated and compared among the three groups.
Results
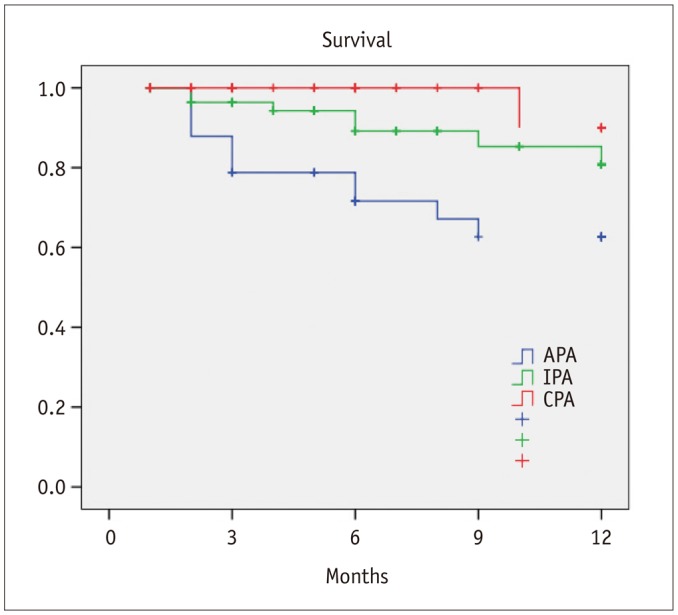
Postprocedural angiography showed the presence of a CPA in 42 patients (30.7%), IPA in 60 patients (43.8%), and APA in 35 patients (25.5%). Healing within 3 months from the procedure was achieved in 21 patients with CPA (50%), 17 patients with IPA (28.3%), and in 7 patients with APA (20%) (p = 0.01). There was a significant difference in terms of 1-year freedom from minor amputation among the three groups (CPA 84.1% vs. IPA 82.4% vs. APA 48.9%, p = 0.001). Estimated 1-year limb salvage was significantly better in patients with CPA (CPA 100% vs. IPA 93.8% vs. APA 70.1%, p < 0.001). Estimated 1-year survival was significantly better in patients with CPA (CPA 90% vs. IPA 80.8% vs. APA 62.7%, p = 0.004).
Diabetes is the main cause of critical limb ischemia (CLI) (12). The worldwide prevalence and incidence of diabetes mellitus are dramatically increasing. In the future, this epidemiological burden may cause a considerable increase in the incidence of CLI in a more elderly diabetic population (3).
In diabetic patients with ischemic foot wounds, vascular involvement is extremely diffuse and aggressive in the below-the-knee arteries with a high percentage of long occlusions (4). These reports suggest that in diabetic patients with foot wounds, the correlation is with “more distal-more aggressive” atherosclerotic disease. Therefore, in the last few years, there has been a great interest not only in the tibial vessels but also in the foot vessels (5).
Even older angiographic studies have demonstrated the importance of evaluating the foot vessels in patients undergoing peripheral revascularization (67). In particular, Ciavarella et al. (7) confirmed more frequent involvement of the tibial and foot vessels in diabetic patients. In addition, it is well known that the status of the foot vessels affects the outcomes of revascularization. Karacagil et al. (8) demonstrated that the pedal runoff may have prognostic significance in femoropopliteal as well as femorodistal bypass surgery, suggesting complete visualization of the tibial and foot arteries before performing a surgical bypass.
More recently, Rashid et al. (9) demonstrated that the pedal arch quality influenced the rates for healing and time to healing, but it did not influence the patency or the amputation-free survival in patients with CLI undergoing surgical infrapopliteal bypass. In the last few years, a lot of papers on endovascular techniques to revascularize the pedal arch were published (101112). Even for the endovascular approach, the majority of studies reported the technical outcomes (procedural success, vessel patency, etc.). Functional and clinical outcomes (wound healing, time to healing, quality of life, functional status, etc.) were only partially discussed.
Our paper seems to be the first to report the clinical implications (wound healing, time to healing, and survival) according to the pedal arch status at the end of an infrainguinal endovascular procedure. The aim of this study was to evaluate, based on our experience, the impact of pedal arch status on tissue loss and time to healing in diabetic patients with CLI undergoing infrainguinal endovascular revascularization.
The study was approved by the Institutional Review Board and all patients gave their written consent for the procedure approved by the Ethics Committee.
Between January 2014 and June 2015, 137 consecutive diabetic patients with foot wounds and CLI underwent infrainguinal endovascular revascularization at our center.
The patients were predominantly men (91 men, 66.4%; 46 women, 33.6%) with a mean age of 74.7 ± 9.7 years. All the patients had tissue loss (Rutherford class 5 [n = 90, 65.7%], Rutherford class 6 [n = 47, 34.3%]). Forty-one patients (29.9%) had ischemic and infected wounds penetrating to bone or joint (Texas University Classification class IIID) (13).
Table 1 summarizes the demographic data. All data concerning the procedures was prospectively collected in a dedicated database with 87 variables. The information included demographics, preoperative risk factors, clinical and diagnostic preoperative assessments, procedural findings, and early (30-day) and follow-up outcomes.
All endovascular interventions were performed in a hybrid operating room under local anaesthesia. The endovascular procedure was tailored to the target vessel that was identified on the basis of preoperative diagnostic assessment by clinical examination of the wound and by means of Duplex scan. Technical success was defined as the restoration of flow in the revascularized infrainguinal main vessel without residual stenosis > 30% or flow-limiting dissection. Patients undergoing revascularization of below-the-ankle vessels were excluded from this study.
Postprocedural angiography of the foot was used to divide the patients into the following three groups according to the postprocedural status of the pedal arch: complete pedal arch (CPA) group, incomplete pedal arch (IPA) group, and absent pedal arch (APA) group.
Complete pedal arch was defined when both the dorsalis pedis artery and at least one of the plantar arteries were patent and joined with each other; IPA was defined when the dorsalis pedis artery or one of the plantar arteries were patent but not joined with each other; and APA was defined when neither the dorsalis pedis artery nor at least one of the plantar arteries were patent but the circulation of the foot was established through collateral vessels (Fig. 1).
The follow-up protocol included medications in an advanced wound care setting (Foot Clinic run by nurses with expertise in wound care), and duplex ultrasound at discharge, 1 month, 6 months, 12 months, and yearly thereafter. Time to healing was defined as the time (expressed in months) needed to achieve complete healing of the wound after the procedure.
Early outcomes in terms of wound healing and time to healing were evaluated and compared among the three groups. The Fisher's exact test was used where appropriate. Continuous data was expressed as the mean ± standard deviation. Categorical data was expressed as percentages. One-year estimated outcomes in terms of freedom from minor amputation (defined as absence of any type of amputation in the lower limb except for toe/ray amputation), limb salvage (defined as absence of above-the-knee or leg amputation), and survival were analyzed and compared among the three groups by life-table analysis (Kaplan-Meier test). Statistical significance was defined at the p < 0.05 level. Statistical analysis was performed using SPSS software (version 18.0 for Windows; SPSS Inc., Chicago, IL, USA).
The main infrainguinal lesion revascularized was located in the tibial artery in 79 cases (57.7%). The mean length of the vascular lesion was 184.6 ± 86.5 mm. In most of the cases (83.9%), the main vascular lesion was an occlusion. In most of the patients (94, 68.6%), two or more vessels were treated. No stent was deployed in the tibial vessels.
Table 2 summarizes the intraprocedural ‘endovascular’ data. Technical success according to the “intention-to-treat” vessel was achieved in 120 cases (87.6%). Intraoperatively, two patients developed acute limb ischemia (in one case due to massive peripheral embolization and in the other case due to acute occlusion of the superficial femoral artery). Furthermore, one patient showed disruption of the distal part of a 0.014-inch guidewire in the anterior tibial artery. No intraoperative access site complication was recorded.
Postprocedural angiography showed the presence of a CPA in 42 patients (30.7%), IPA in 60 patients (43.8%), and APA in 35 patients (25.5%).
The three groups were homogeneous, except for a higher number of diabetic patients under insulin treatment in the APA group. Furthermore, tibial revascularization was widely performed in patients with APA. Table 3 shows the comparative data.
During the hospital stay (mean duration, 4.1 ± 3.3 days), all patients underwent aggressive wound management. Surgical treatment of the wound consisted of debridement without bone resection in 47 cases (34.3%), toe/ray amputation in 12 cases (8.7%), Lisfranc amputation in 1 case (0.7%), and transmetatarsal amputation in 6 cases (4.4%).
At 30 days, 2 patients died, resulting in an overall 30-day mortality rate of 1.5%. Furthermore, 9 major systemic complications occurred (1 case of acute respiratory failure, 2 cases of acute heart failure, 2 case of myocardial infarction, and 4 cases of sepsis), and 5 patients underwent leg amputation. The 30-day overall major morbidity rate was 10.2%, while the 30-day major amputation rate was 3.6%. The causes of major amputation were sepsis (4 cases) and massive necrosis of the foot (1 case).
During the follow-up (mean duration, 7.4 ± 4.6 months), complete healing of the lesions was achieved in 35 patients (83.3%) with CPA, 34 patients (56.7%) with IPA, and in 13 patients (37.1%) with APA (p < 0.001). The overall mean time required to achieve wound healing was 4.4 ± 3.5 months. In the CPA, IPA, and APA groups the mean time to healing was 3.5 ± 2.2 months, 4.5 ± 3.1 months, and 5.7 ± 4.4 months, respectively (p < 0.001).
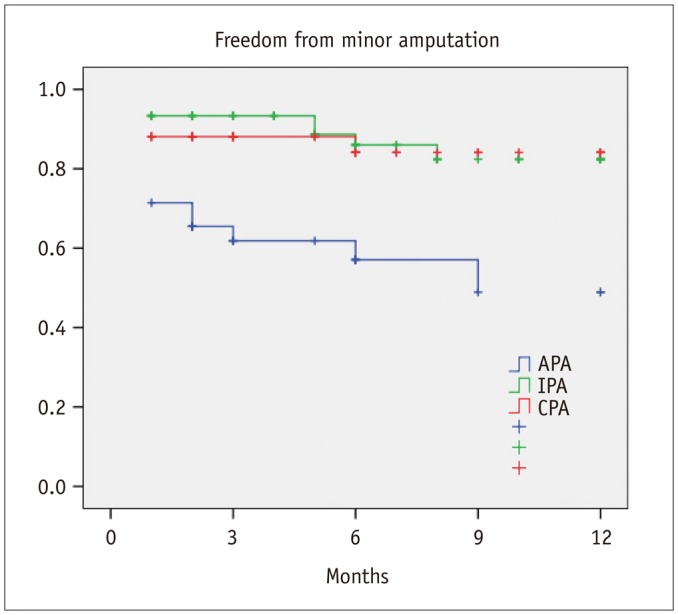
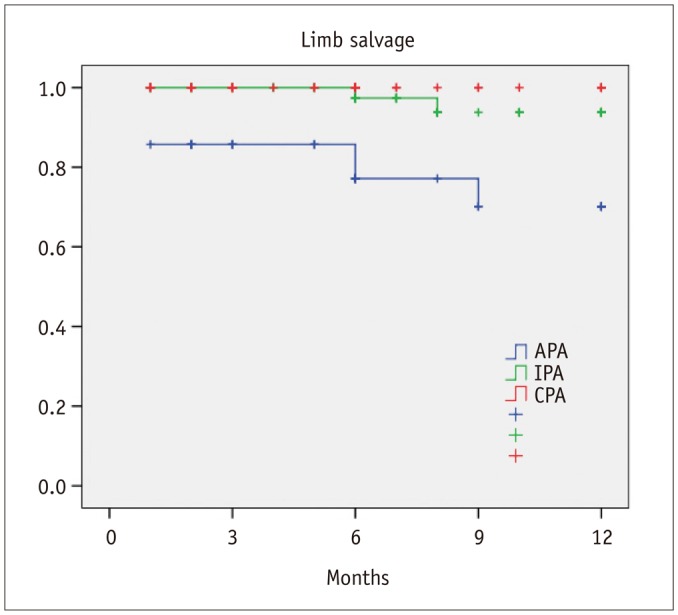
Healing within 3 months from the procedure was achieved in 21 patients with CPA (50%), 17 patients with IPA (28.3%), and in 7 patients with APA (20%) (p = 0.01). In 6 patients (4.4%), the application of a Vacuum Assisted Therapy (VAC; KCI Inc., San Antonio, TX, USA) was necessary. There was a significant difference in terms of 1-year freedom from minor amputation among the three groups (CPA, 84.1%; IPA, 82.4%; APA, 48.9%; p = 0.001) (Fig. 2). Furthermore, estimated 1-year limb salvage was significantly better in patients with CPA (CPA, 100%; IPA, 93.8%; APA, 70.1%; p < 0.001) (Fig. 3). Finally, estimated 1-year survival was significantly better in patients with CPA (CPA, 90%; IPA, 80.8%; APA, 62.7%; p = 0.004) (Fig. 4).
Since the 1980s, the importance of evaluation of the pedal arch in patients with CLI has been well established. Lea Thomas et al. (14) performed a foot angiography in 100 consecutive patients with ‘leg ischemia’; they demonstrated that the plantar arch was present in 75% of feet, occluded in 12% of feet, and not demonstrated for technical reasons in 13% of feet. They concluded that it is always recommended to make an attempt to demonstrate the patency of the plantar arch in order to assess the probability of patency of a distal bypass graft.
Seven years before O'Mara et al. (15) demonstrated the angiographic correlation of pedal arch patency with early tibial bypass patency; six of the seven grafts failed in patients with an occluded pedal arch. In 1990s, there was a great interest in open surgical revascularization of foot arteries in patients with CLI. Gloviczki et al. (16) demonstrated that pedal bypass is a safe, effective, and durable procedure, and they suggested its use even in high-risk patients with CLI before major amputation. Furthermore, Davidson and Callis (17) reported the outcomes of 75 bypasses to the arteries of the foot and ankle, which demonstrated that it was possible to perform an aggressive surgical approach in patients with CLI with occluded below the knee vessels and a patent pedal arch so as to maintain a functional extremity in the majority of the patients.
During this period, the majority of studies reported the outcomes in terms of patency and demonstrated the correlation between patency of the pedal arch and patency of open surgical revascularization. In the 2000s, there was growing attention to the pedal arch patency in diabetic and non-diabetic patients with CLI. This was related to the epidemic diffusion of the endovascular procedures and the concomitant improvement in techniques and materials.
Manzi et al. (10) reported the clinical outcomes obtained in 135 patients with CLI who were treated by balloon angioplasty of the foot vessels by using the pedal-plantar loop technique; immediate success evaluated with a significant improvement in the transcutaneous oxygen tension was maintained at one year in the majority of the patients. In the last few years, ancillary techniques were added to the standard ‘antegrade’ approach. Some papers have summarized the tips and tricks for these techniques (11). In particular, the antegrade pedal approach seems to be useful in recanalizing an occluded pedal arch when retrograde puncture is not possible (12). Even for the endovascular approach, the majority of studies have reported the technical outcomes (procedural success, vessel patency, etc.). Functional and clinical outcomes (wound healing, time to healing, quality of life, functional status, etc.) were only partially discussed.
In our study, diabetic patients with CPA/IPA seemed to have better outcomes in terms of wound healing and limb salvage. Surprisingly, the pedal arch patency even affected the survival of diabetic patients. In fact, pedal arch patency was found to be associated with overall survival of diabetic patients, suggesting that it may be indicative of a more advanced atherosclerotic process. Furthermore, patients with a poorer pedal arch have been found to have more aggressive diabetes (a higher percentage of patients under insulin treatment). Our paper seems to be the first to report the clinical implications (wound healing, time to healing, and survival) according to the pedal arch status at the end of an infrainguinal endovascular procedure.
Recently, Rashid et al. (9) reported the clinical impact of pedal arch quality and angiosome revascularization in 154 patients with CLI who had undergone 167 open surgical infrapopliteal bypasses; they demonstrated that there was a significant difference in wound healing and time to healing between the patients with CPA/IPA and no pedal arch. Furthermore, they reported a high percentage of wound healing in patients with CPA/IPA, independently of the angiosome revascularized.
The outcomes of our study in a diabetic population that underwent peripheral endovascular revascularization were similar to those obtained by Rashid et al. (9). In fact, in our study complete wound healing was achieved in 35 patients with CPA (83.3%), 34 patients with IPA (56.7%), and in 13 patients with APA (37.1%) with a significant difference (p < 0.001). Furthermore, in our subgroup analysis, wound healing seemed to be unaffected by aggressive endovascular treatment of the tibial vessels (more tibial revascularizations in patients with APA, but lower wound healing rates). In addition, our data demonstrated that pedal arch status affected limb salvage and survival rate. The data about survival were quite surprising and were not previously reported. Therefore, pedal arch status seemed to be a positive prognostic marker for limb salvage and survival in diabetic patients with CLI. More recently, Nakama et al. (1819) emphasized the importance of an additional pedal artery angioplasty in order to improve the wound blush and clinical outcomes (in particular, speed and extent of wound healing). In particular, the multicenter RENDEZVOUS registry (19) demonstrated that patients who underwent pedal artery angioplasty showed a higher rate of wound healing and shorter time to wound healing, especially in the moderate-risk population.
This study has some limitations. First of all, it is a retrospective analysis of a selected diabetic population with ischemic foot wounds who underwent peripheral endovascular treatment and it is not a global analysis of diabetic patients. This should be considered as a selection bias. Second, the study population was small; further analyses in a larger population study and a longer follow-up are needed. Third, clinical outcomes in diabetic patients with foot wounds are commonly affected by the efficiency of a multidisciplinary diabetic foot program. A high degree of cooperation between all the professionals involved in the clinical care pathways is necessary to achieve wound healing and limb salvage. Fourth, the study group is heterogeneous, with varying segments of treatment and limited information about lesion characteristics.
In conclusion, pedal arch patency has a positive clinical impact on time to healing, limb salvage, and survival in diabetic patients with CLI undergoing infrainguinal endovascular revascularization.
References
1. Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999; 22:382–387. PMID: 10097914.

2. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005; 293:217–228. PMID: 15644549.

3. Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005; 366:1719–1724. PMID: 16291066.

4. Graziani L, Silvestro A, Bertone V, Manara E, Andreini R, Sigala A, et al. Vascular involvement in diabetic subjects with ischemic foot ulcer: a new morphologic categorization of disease severity. Eur J Vasc Endovasc Surg. 2007; 33:453–460. PMID: 17196848.

5. Manzi M, Cester G, Palena LM, Alek J, Candeo A, Ferraresi R. Vascular imaging of the foot: the first step toward endovascular recanalization. Radiographics. 2011; 31:1623–1636. PMID: 21997985.

6. Karacagil S, Almgren B, Bowald S, Eriksson I. Arterial lesions of the foot vessels in diabetic and non-diabetic patients undergoing lower limb revascularisation. Eur J Vasc Surg. 1989; 3:239–244. PMID: 2744155.

7. Ciavarella A, Silletti A, Mustacchio A, Gargiulo M, Galaverni MC, Stella A, et al. Angiographic evaluation of the anatomic pattern of arterial obstructions in diabetic patients with critical limb ischaemia. Diabete Metab. 1993; 19:586–589. PMID: 8026611.
8. Karacagil S, Almgren B, Lörelius LE, Bowald S, Eriksson I. Angiographic runoff patterns in patients undergoing lower limb revascularization. Acta Chir Scand. 1989; 155:19–24. PMID: 2929199.
9. Rashid H, Slim H, Zayed H, Huang DY, Wilkins CJ, Evans DR, et al. The impact of arterial pedal arch quality and angiosome revascularization on foot tissue loss healing and infrapopliteal bypass outcome. J Vasc Surg. 2013; 57:1219–1226. PMID: 23523278.

10. Manzi M, Fusaro M, Ceccacci T, Erente G, Dalla Paola L, Brocco E. Clinical results of below-the knee intervention using pedalplantar loop technique for the revascularization of foot arteries. J Cardiovasc Surg (Torino). 2009; 50:331–337.
11. Gandini R, Del Giudice C, Simonetti G. Pedal and plantar loop angioplasty: technique and results. J Cardiovasc Surg (Torino). 2014; 55:665–670.
12. Palena LM, Manzi M. Antegrade pedal approach for recanalizing occlusions in the opposing circulatory pathway of the foot when a retrograde puncture is not possible. J Endovasc Ther. 2014; 21:775–778. PMID: 25453877.

13. Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998; 21:855–859. PMID: 9589255.

14. Lea Thomas M, Tanqueray AB, Burnand KG. Visualization of the plantar arch by aortography: technique and value. Br J Radiol. 1988; 61:469–472. PMID: 3370428.
15. O'Mara CS, Flinn WR, Neiman HL, Bergan JJ, Yao JS. Correlation of foot arterial anatomy with early tibial bypass patency. Surgery. 1981; 89:743–752. PMID: 7245037.
16. Gloviczki P, Bower TC, Toomey BJ, Mendonca C, Naessens JM, Schabauer AM, et al. Microscope-aided pedal bypass is an effective and low-risk operation to salvage the ischemic foot. Am J Surg. 1994; 168:76–84. PMID: 8053532.

17. Davidson JT 3rd, Callis JT. Arterial reconstruction of vessels in the foot and ankle. Ann Surg. 1993; 217:699–708. discussion 708-710. PMID: 8507116.

18. Nakama T, Watanabe N, Kimura T, Ogata K, Nishino S, Furugen M, et al. Clinical implications of additional pedal artery angioplasty in critical limb ischemia patients with infrapopliteal and pedal artery disease. J Endovasc Ther. 2016; 23:83–91. PMID: 26442951.

19. Nakama T, Watanabe N, Haraguchi T, Sakamoto H, Kamoi D, Tsubakimoto Y, et al. Clinical outcomes of pedal artery angioplasty for patients with ischemic wounds: results from the multicenter RENDEZVOUS registry. JACC Cardiovasc Interv. 2017; 10:79–90. PMID: 28057289.
Fig. 1
Pedal arch status on postprocedural angiography: CPA (A), IPA (B), APA (C).
APA = absent pedal arch, Arch = pedal arch, CPA = complete pedal arch, IPA = incomplete pedal arch, PedA = pedis artery, PlaA = plantar artery

Table 1
Overall Demographic Data and Preoperative Risk Factors of Patients

Table 2
Intraprocedural ‘Endovascular’ Data of Patients
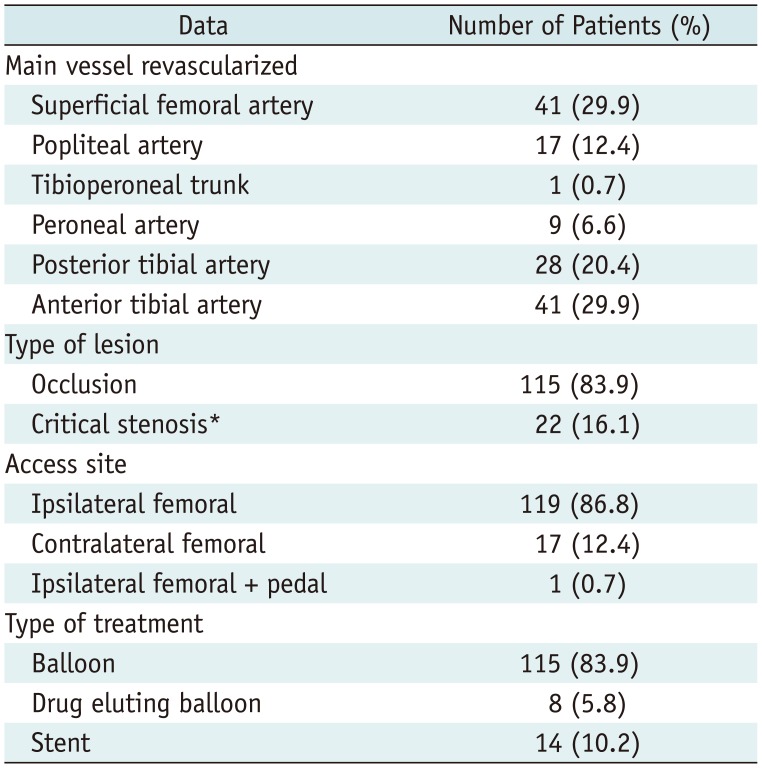
Table 3
Demographic and Intraprocedural Data: Comparison of Three Groups




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download