INTRODUCTION
Hepatic alveolar echinococcosis (HAE) is caused by the parasitic cestode
Echinococcus multilocularis (
E. multilocularis), which is endemic in many parts of the world. Without timely diagnosis and therapy, the prognosis is dismal–the eventual outcome in most cases is death. Surgical resection of primary lesions allows curative treatment. However, more than 70% of cases are inoperable at the time of diagnosis. In these cases, there is a tumor-like growth of the parasite with diffuse infiltration of “non-resectable” structures, or insufficient safety margins. In such cases, long-term or even life-long pharmacological treatment with benzimidazoles would be necessary (
12).
It is of great importance, therefore, to employ imaging techniques that can detect and monitor the viability of inoperable HAE, which would help optimize therapeutic management. For instance, detection of dead parasites permits the duration of chemotherapy to be shortened. Likewise, for those with persistent or increasing disease activity, long-term medical treatment or other intervention is necessary. However, HAE viability cannot be obtained by conventional imaging techniques (i.e., ultrasound [US], computed tomography [CT], and MRI).
Previous studies have demonstrated the value of 18-fluoro-deoxyglucose positron emission tomography computed tomography (18F-FDG PET/CT) in assessing the viability of HAE. This imaging procedure is currently considered the best surrogate marker of parasite viability (
34). Despite its value, PET/CT has several notable disadvantages. These include the need to use high radiation doses, tedious preparation procedures for 18F-FDG and the patient, and the lengthy time required for FDG uptake and scanning. In addition, PET/CT scanners are not as widely available as CT or MRI scanners.
To the best of our knowledge, diffusion-weighted imaging (DWI) sequence provides anatomical and functional details is rarely used in the assessment of HAE viability (
56). However, we wondered it could be used without the notable disadvantages found with PET/CT. The aim of this study thus explores the value of DWI in assessing the viability of HAE by comparing its performance to that of PET/CT.
Go to :

MATERIALS AND METHODS
Patients
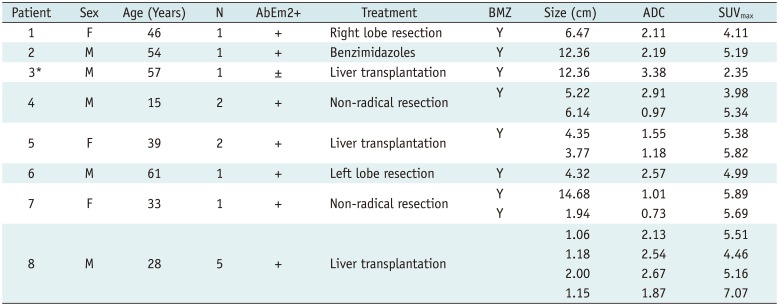
Eight patients with HAE were evaluated retrospectively at the first hospital of Xinjiang Medical University, including three females and five males. Institutional Review Board approval and the requirements for informed consent were obtained for all patients involved in this study. The age range of the patients was 15–58 years (mean age, 40.5 years). The diagnosis of HAE had been established by patients' pastoral life history, serology (specific antibodies against E. multilocularis with the Em2plus- enzyme-linked immune sorbent assay [ELISA]), and typical imaging findings (US, CT and MRI). Seven patients were evaluated for an initial diagnosis; one was diagnosed with HAE and had received oral drug therapy for three years. Eight patients had MRI and PET/CT examination within one week prior to each study.
MRI and DWI Acquisition
For image acquisition, the same protocol was adopted for all examinations using a clinical 3T MR system (Signa Exite HD; GE Healthcare, Milwaukee, WI, USA) with a eight-channel phased-array torso surface coil. The conventional MRI protocol consisted of transverse unenhanced T1-weighted images (T1WI) and respiratory-triggered T2-weighted images (T2WI). A respiratory-triggered single shot fat suppressed echo planar DWI sequence was acquired with the gradient factors (b-values were 0 and 800 s/mm2). Consequently, the corresponding apparent diffusion coefficient (ADC) map was generated using ADW 4.2 scanner software (GE Healthcare).
FDG-PET/CT Image Acquisition
All PET/CT examinations were performed on a PET/CT scanner (Discovery VCT; GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA). All patients fasted for at least eight hours before the administration of an injection containing FDG at 4.8 MBq/kg body weight. PET/CT scans were performed 60 minutes after the injection of the FDG. A whole-body emission PET scan with a 70-cm axial field of view, a 218 x 218 matrix, a slice thickness of 4.25 mm was performed with five bed positions within 20 minutes. The PET and CT fusion images were obtained by image reconstruction in cross, coronal, and sagittal sections.
Image Analysis and Statistical Analysis
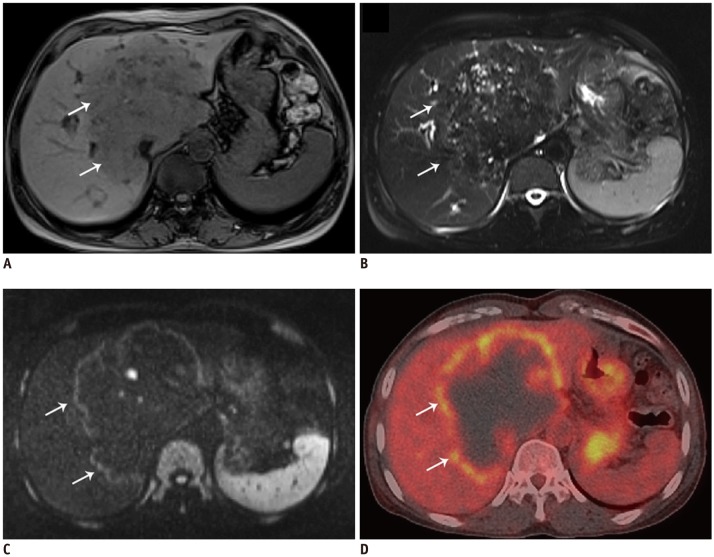
Two different radiologists worked independently to determine the activity of HAE lesions. They used the following standard: (+) was used to mark focally or perilesionally increased FDG uptake/hyper-signal intensity. Conversely, (−) was used to mark a hepatic defect without FDG uptake/no hyper-signal intensity, on PET/CT and DWI respectively.
The region-of-interest (ROI) was placed on fused PET/CT, covering the metabolic activity area. Then, we performed a semi-quantitative analysis by extracting the maximum standardized uptake value (SUVmax) from each lesion's PET images, defined as maximum SUV value within the delineated viability area. Also, a polygonal ROI was placed manually on every ADC slice encompassing the entire viability area appearing as an area of low signal intensity. We calculated mean ADC value within all ADC slices displaying the metabolic activity area.
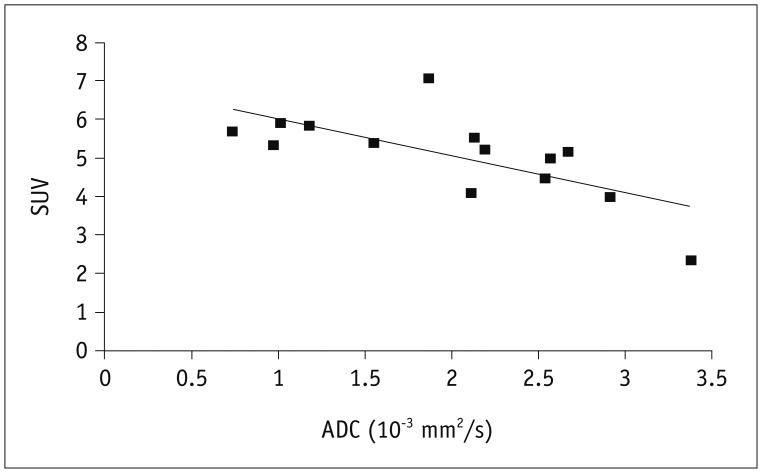
We performed our statistical analysis using the SPSS software package (version 18.0; SPSS Inc., Chicago, IL, USA). We used Pearson's correlation coefficient to determine the relationship of ADC and SUVmax data and p values < 0.01 were considered significant.
Go to :

DISCUSSION
MRIs, with its conventional sequences, such as T1WI, T2WI and enhanced T1-weighted sequences, have been used for the diagnosis of HAE and the corresponding complications in previous studies (
78). While DWI had only been utilized in one previous study, it had demonstrated its value. DWI was able to illuminate characteristics of HAE lesions according to Kodama's classification system; however, no mention was made on its role in viability in this research (
9).
Diffusion-weighted imaging is based on the physical process of random Brownian motion of water molecules in the body. Diffusion of intracellular water molecules is restricted by the presence of cell and organelle membranes. In highly-cellular tissues, such as neoplasm, diffusion is restricted due to the smaller extra-cellular volume and higher density of hydrophobic cellular membranes. There is good evidence that DWI has the potential to improve lesion detection rates and contribute to the evaluation and characterization of hepatic lesions (
1011).
In the current research, an association was demonstrated between DWI and PET/CT in depicting the metabolic activity of HAE, in terms of both their intensity and distribution. Previous oncologic studies would serve to confirm this phenomenon.
For example, DWI showed a close distribution with PET/CT for the evaluation of malignancy of lymph node metastasis in the head and neck, indicating the existence of a relationship between the density and metabolic activity of tumor cells (
12).
Hepatic alveolar echinococcosis grows multifocally and invasively, with protrusions of its germinal layer into the host tissue causing an intense inflammatory reaction, granuloma formation, and increased radiotracer uptake on the FDG-PET-CT (
1314). Restricted water diffusion caused by inflammatory cell infiltration can also be detected with DWI. Therefore, the same nature of HAE inflammatory granuloma was disclosed from different aspects via two technologies. In a comparative study of malignant tumors, a correlation analysis was conducted between the respective quantitative indicators, the ADC and the SUV of DWI and PET/CT (
15).
In this study, we found a significant inverse correlation between the SUVmax and the ADC. This correlation, to a certain degree, supports the assumption that DWI might be of value in assessing the viability of HAE as PET/CT does.
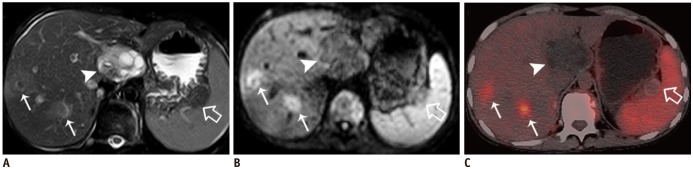
As mentioned earlier, in one of our cases, a patient underwent oral drug therapy for three years. For this patient, the perilesional high-signal intensity in the DWI was still present but significantly decreased, while the PET/CT scans showed a hepatic defect without any FDG uptake. This was only the discrepancy between PET/CT and DWI in our study. We attribute the discrepancy as possibly being caused by a false-positive DWI, or false-negative PET/CT. Criteria of cure for a patient with an inoperable lesion (that is, criteria for non-viable lesion) are still unsettled, but should include a negative PET/CT scan, calcified component of the alveolar echinococcosis lesion of more than 50% as well as the disappearance of specific antibodies (
1). If based on this criteria, this treated case should still have had a viable lesion (a negative PET/CT, while no obvious calcification, equivocal specific antibodies).
It was reported that PET negativity does not necessarily indicate non-activity of the parasite (
13). After chemotherapy, the HAE metabolic activity can be too low for detection with PET, and/or positivity of very small peripheral foci may be missed due to the limited spatial resolution (5 mm) of the PET scanner. Due to our limited experience and without any other study references, we cannot conclude that DWI is more sensitive in detecting parasitic vitality after benzimidazole therapy than PET/CT does. However, whether DWI is more sensitive may be worth exploring in further studies.
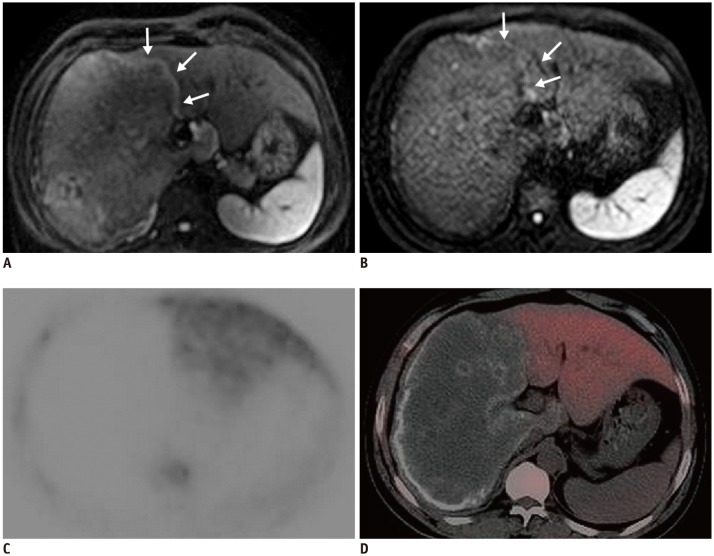
In our study, five small lesions (< 2 cm in diameter) presented as increased nodular metabolic activity both on DWI and PET/CT due to multiple granulomas fused together. In the larger lesions, the central part without any viability consisted of fibrotic tissue, coagulation necrosis, liquefaction necrosis and/or calcifications. The activity was distributed in a pattern of central “filling defects” and lateral “hot spots,” which mainly existed in the lesion's border with normal liver parenchyma. While a lesion will show activity reduction or inactivity in the liver capsular and diaphragm area (
341316), in most cases,
E. multilocularis will develop parasitic activity characterized by unidirectional growth. Therefore, one extreme of a parasitic lesion may show metabolic activity while the polar opposite consists of necrotic tissue, which is why fine-needle biopsy is not recommended as a conventional viability assessing technique (
4).
Even knowing this, it is still hard to explain the phenomenon that inactivity is always found near the liver capsular and diaphragm area. Could it be said that those fibrous membranes play some role in stopping the development, or even gradually causing partial death of the parasite? Whether this so-called “contact trigger death” exists or not requires further research.
Prior to making our conclusions, we note the limitations of this study. Particularly, we note that the overall number of included patients (eight) was small, and only one patient underwent follow-up repeated imaging. Therefore, we may only conclude that DWI and PET/CT may agree well in terms of characterizing initial active HAE, and their agreement on treated or inactive HAE needs further study.
In conclusion, DWI is comparable to PET/CT in visually detecting the viability of HAE lesion. Significant inverse correlations were found between ADC from DWI and SUVmax from PET/CT on data of HAE lesions. This finding indicates that DWI is potentially capable of offering similar information for evaluating metabolic activity in HAE patients as well as PET/CT does.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download