1. Azizad S, Sannananja B, Restrepo CS. Solid tumors of the mediastinum in adults. Semin Ultrasound CT MR. 2016; 37:196–211. PMID:
27261345.

2. Shepherd A, Riely G, Detterbeck F, Simone CB 2nd, Ahmad U, Huang J, et al. Thymic Carcinoma Management Patterns among International Thymic Malignancy Interest Group (ITMIG) physicians with consensus from the Thymic Carcinoma Working Group. J Thorac Oncol. 2017; 12:745–751. PMID:
27876674.

3. Piña-Oviedo S, Moran CA. Primary mediastinal classical hodgkin lymphoma. Adv Anat Pathol. 2016; 23:285–309. PMID:
27441757.

4. Abdel Razek AA, Soliman N, Elashery R. Apparent diffusion coefficient values of mediastinal masses in children. Eur J Radiol. 2012; 81:1311–1314. PMID:
21439745.

5. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845. PMID:
3203132.

6. Abdel Razek AA, Khairy M, Nada N. Diffusion-weighted MR imaging in thymic epithelial tumors: correlation with World Health Organization classification and clinical staging. Radiology. 2014; 273:268–275. PMID:
24877982.

7. Gümüştaş S, Inan N, Sarisoy HT, Anik Y, Arslan A, Ciftçi E, et al. Malignant versus benign mediastinal lesions: quantitative assessment with diffusion weighted MR imaging. Eur Radiol. 2011; 21:2255–2260. PMID:
21698463.

8. Shin KE, Yi CA, Kim TS, Lee HY, Choi YS, Kim HK, et al. Diffusion-weighted MRI for distinguishing non-neoplastic cysts from solid masses in the mediastinum: problem-solving in mediastinal masses of indeterminate internal characteristics on CT. Eur Radiol. 2014; 24:677–684. PMID:
24177751.

9. Seki S, Koyama H, Ohno Y, Nishio M, Takenaka D, Maniwa Y, et al. Diffusion-weighted MR imaging vs. multi-detector row CT: direct comparison of capability for assessment of management needs for anterior mediastinal solitary tumors. Eur J Radiol. 2014; 83:835–842. PMID:
24636535.

10. Yabuuchi H, Matsuo Y, Abe K, Baba S, Sunami S, Kamitani T, et al. Anterior mediastinal solid tumours in adults: characterisation using dynamic contrast-enhanced MRI, diffusion-weighted MRI, and FDG-PET/CT. Clin Radiol. 2015; 70:1289–1298. PMID:
26272529.
11. Xu X, Su G, Hu H, Wang Y, Hong X, Shi H, et al. Effects of regions of interest methods on apparent coefficient measurement of the parotid gland in early Sjögren's syndrome at 3T MRI. Acta Radiol. 2017; 58:27–33. PMID:
26987670.

12. Zhang YD, Wang Q, Wu CJ, Wang XN, Zhang J, Liu H, et al. The histogram analysis of diffusion-weighted intravoxel incoherent motion (IVIM) imaging for differentiating the gleason grade of prostate cancer. Eur Radiol. 2015; 25:994–1004. PMID:
25430007.

13. Lu SS, Kim SJ, Kim N, Kim HS, Choi CG, Lim YM. Histogram analysis of apparent diffusion coefficient maps for differentiating primary CNS lymphomas from tumefactive demyelinating lesions. AJR Am J Roentgenol. 2015; 204:827–834. PMID:
25794073.

14. Xu XQ, Hu H, Su GY, Zhang L, Liu H, Hong XN, et al. Orbital indeterminate lesions in adults: combined magnetic resonance morphometry and histogram analysis of apparent diffusion coefficient maps for predicting malignancy. Acad Radiol. 2016; 23:200–208. PMID:
26625705.
15. Choi MH, Oh SN, Rha SE, Choi JI, Lee SH, Jang HS, et al. Diffusion-weighted imaging: apparent diffusion coefficient histogram analysis for detecting pathologic complete response to chemoradiotherapy in locally advanced rectal cancer. J Magn Reson Imaging. 2016; 44:212–220. PMID:
26666560.

16. Nougaret S, Vargas HA, Lakhman Y, Sudre R, Do RK, Bibeau F, et al. Intravoxel incoherent motion-derived histogram metrics for assessment of response after combined chemotherapy and radiation therapy in rectal cancer: initial experience and comparison between single-section and volumetric analyses. Radiology. 2016; 280:446–454. PMID:
26919562.

17. Xu XQ, Hu H, Su GY, Liu H, Hong XN, Shi HB, et al. Utility of histogram analysis of ADC maps for differentiating orbital tumors. Diagn Interv Radiol. 2016; 22:161–167. PMID:
26829400.

18. Razek AA, Elkhamary S, Mousa A. Differentiation between benign and malignant orbital tumors at 3-T diffusion MR-imaging. Neuroradiology. 2011; 53:517–522. PMID:
21286695.

19. Takahashi K, Al-Janabi NJ. Computed tomography and magnetic resonance imaging of mediastinal tumors. J Magn Reson Imaging. 2010; 32:1325–1339. PMID:
21105138.

20. Donati OF, Mazaheri Y, Afaq A, Vargas HA, Zheng J, Moskowitz CS, et al. Prostate cancer aggressiveness: assessment with whole-lesion histogram analysis of the apparent diffusion coefficient. Radiology. 2014; 271:143–152. PMID:
24475824.

21. Suo S, Zhang K, Cao M, Suo X, Hua J, Geng X, et al. Characterization of breast masses as benign or malignant at 3.0T MRI with whole-lesion histogram analysis of the apparent diffusion coefficient. J Magn Reson Imaging. 2016; 43:894–902. PMID:
26343918.

22. Johnson PW, Davies AJ. Primary mediastinal B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2008; 349–358. PMID:
19074109.

23. Ströbel P, Bauer A, Puppe B, Kraushaar T, Krein A, Toyka K, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol. 2004; 22:1501–1509. PMID:
15084623.

24. Xu XQ, Choi YJ, Sung YS, Yoon RG, Jang SW, Park JE, et al. Intravoxel incoherent motion MR imaging in the head and neck: correlation with dynamic contrast-enhanced MR imaging and diffusion-weighted imaging. Korean J Radiol. 2016; 17:641–649. PMID:
27587952.

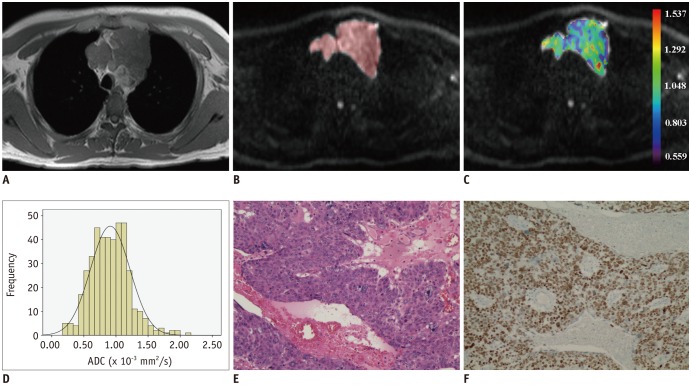
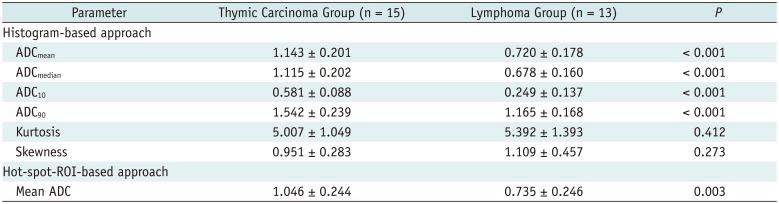
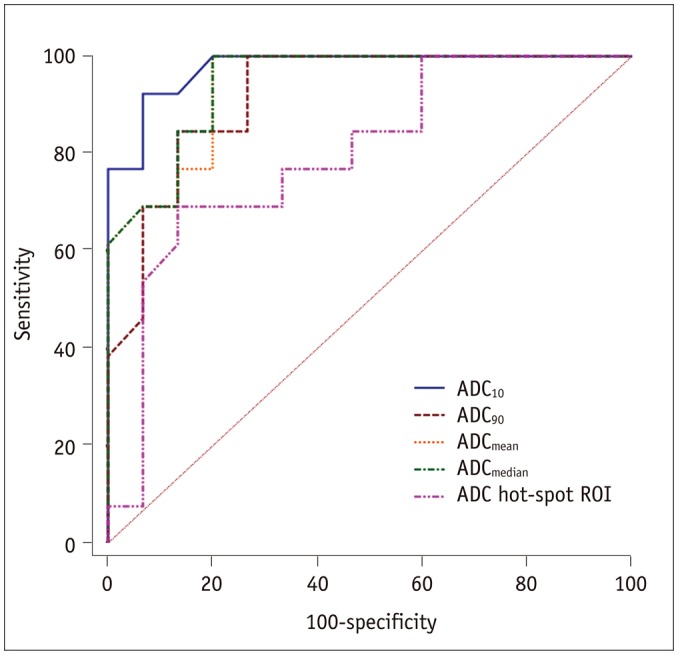
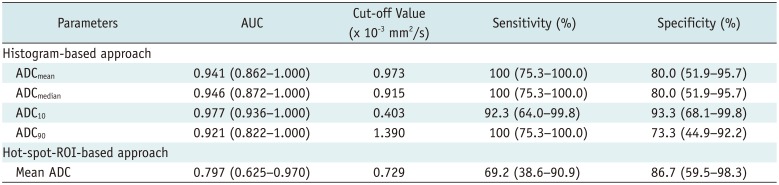




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download