Abstract
Objective
This study assessed the risk of acute allergic-like reactions (AARs) after extravascular administration of iodinated contrast media (ICM) in at-risk patients compared with that after intravascular ICM administration.
Materials and Methods
From July 2012 to January 2016, 264 patients with a history of moderate or severe reactions to ICM, with re-exposure to ICM intravascularly or extravascularly were included. The incidence of recurrent AARs after ICM re-exposure were assessed according to the administration routes by reviewing electronic medical records and comparison between the two routes.
Results
Among 264 patients, 244 patients had been subsequently exposed to ICM intravascularly, 7 patients via an extravascular route and 13 patients with dual re-exposure. Of 257 patients with intravascular ICM re-exposure, 87 (33.9%) had mild to severe recurrent AARs and 143 (19.5%) cases of recurrent AARs occurred among 733 cases of intravascular ICM re-exposure on a case-by-case basis. However, there was no case of recurrent ARR after extravascular administration of ICM in 20 patients (45 cases) with ICM administrated extravascularly.
Iodinated contrast media (ICM) are used in different types of radiologic examinations to enhance tissue contrast and improve lesion detectability and characterization (12). The most common and important effect related to ICM is an acute allergic-like reaction (AAR), which is defined as an adverse reaction occurring within one hour after contrast media injection (34). Most AARs are minor and usually no treatment is required (256). Rarely, fatal anaphylaxis can occur (78). Previous AAR to ICM is the most important risk factor for recurrent reactions upon subsequent exposure to ICM (8910).
Although most AARs occur after intravascular administration of ICM, reactions related with extravascular usage of ICM have also been reported (3111213). Even though AARs developing after extravascular usage of ICM were described in several case reports (111415), the exact incidence of AARs after extravascular administration of ICM is unclear. It is almost impossible to determine the incidence because of the difficulty in acquiring sufficient number of AAR cases upon extravascular usage of ICM.
Determining the risk of extravascular ICM usage by comparing it with the risk of intravascular ICM usage would be a valuable and realistic method for estimating the risk of AAR upon extravascular usage of ICM. The purpose of this study was to reveal the risk of AARs after extravascular administration of ICM in at-risk patients compared with that after intravascular ICM administration.
The Institutional Review Board of the Seoul National University Hospital approved this retrospective study. The requirement for informed consent was waived.
The Contrast Safety Monitoring and Management electronic medical record system was initiated in July 2012. All symptoms suggestive of AARs to ICM were mandated to be monitored and recorded in real-time by trained nurses for all radiologic examinations performed at our institution (9). When patients with a previous history of AARs to ICM were re-exposed to ICM, occurrence or absence of AARs was also recorded. The system adopted three severity categories (mild, moderate, and severe) based on the American College of Radiology manual on contrast media (3). In this management system, premedication regimens determined by the severity of the prior AARs are automatically proposed to the ordering physicians, when patients with a history of immediate ICM allergic-like reactions are scheduled to undergo contrast-enhanced computed tomography examination (9).
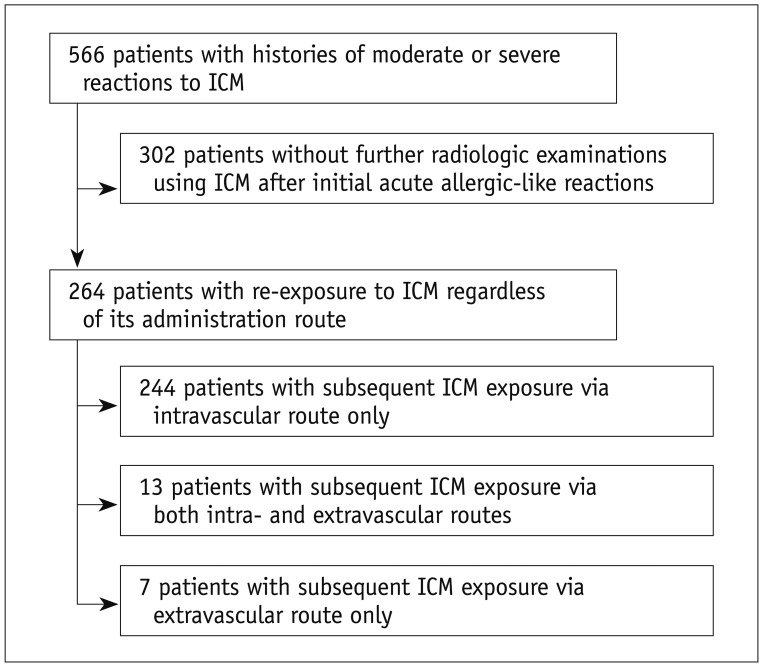
The study extracted data in the database generated from the July 2012 to January 2016. The data included details of all (n = 566) patients with histories of moderate or severe reactions to ICM who had previously experienced moderate to severe reactions to intravascularly administrated ICM. There were no patients with a history of moderate or severe reactions to extravascularly administered ICM. Among the 566 patients, 302 did not receive further radiologic examinations using ICM after initial AARs. They were excluded. Finally, 264 patients with histories of moderate or severe reactions to ICM and re-exposure to ICM regardless of its administration route comprised the study cohort. Data including age, sex, severity of index AARs to ICM, and the types of culprit contrast agents categorized according to the relative osmolality were collected from the same system. Number of re-exposures to ICM according to routes of ICM administration (intravascular vs. extravascular), time interval between the index AAR event and the first re-exposure to ICM, and the types of re-exposed contrast media according to the relative osmolality were evaluated by review of the electronic medical records. Extravascular ICM administration included ICM administration into gastrointestinal (GI), genitourinary (GU), or pancreatico-biliay tracts, pleural or peritoneal space, and cerebrospinal fluid space.
Electronic medical records of radiologic examinations using ICM via an extravascular route were reviewed to determine whether prophylaxis was given before the examinations or not. To prevent missing cases, a radiologist reviewed the electronic medical records of all relevant patients and searched cases to identify suspicious symptoms that developed after extravascular administration of ICM. An experienced allergist and radiologist subsequently reviewed the cases to determine whether symptoms were AARs to ICM.
Recurrent AAR rates were evaluated on per patient and per case basis. The difference between intravascular and extravascular administrations was evaluated with descriptive analysis. The Fisher's exact test was used to compare demographic characteristics and recurrent AAR rates between two routes. Statistical analyses were performed using SPSS software version 21.0 for Windows (IBM Corp., Armonk, NY, USA).
Among the 264 patients (median age, 57 years; range, 8–87 years; 108 males and 156 females) with a history of moderate to severe reactions, 244 patients were subsequently exposed to ICM through an intravascular route only, 7 patients through an extravascular route only and 13 patients through both routes (Fig. 1). Accordingly, 257 patients (median age, 57 years; range, 8–87 years; 105 males and 152 females) were re-exposed to ICM via the intravascular route and 20 patients (median age, 59 years; range, 45–70 years; 12 males and 8 females) were re-exposed via the extravascular route (Table 1). For all 264 patients, the type of culprit ICMs for index AAR administered via the intravascular route was low-osmolar contrast media (LOCM) (Table 1).
Twenty patients with moderate (n = 19) and severe (n = 1) index AARs underwent a total of 45 radiologic examinations with extravascular ICM administration (Table 2). ICM was administrated into the GI tract (n = 6), GU tract (n = 7), pancreatico-biliary tract (n = 12), cerebrospinal fluid space (n = 6) and the abdominal cavity (n = 14). The number of extravascular re-exposures to ICM per person ranged from 1 to 9 (median, 1). Time interval between the index AAR and the first re-exposure to ICM via an extravascular route was from 1 to 835 days (median day, 267 days). High-osmolar contrast media (HOCM) were used in 39 cases, with diatrizoate meglumine and diatrizoate sodium (Gastrografin®; Bristol-Myers Squibb, Princeton, NJ, USA) used in 5 cases and ioxithalamate (Telebrix®; Laboratoire Guerbet, Paris, France) in 34 cases. LOCM were used in 6 cases, with iohexol (Omnipaque®; GE Healthcare, Milwaukee, WI, USA) used in all cases. In two of six cases using iohexol, the culprit ICM was also iohexol. No patient received premedication before examinations.
There were two cases where suspicious symptoms had occurred after extravascular administration of ICM. One patient had severe hypotension (79/52 mm Hg) 2 hours after the percutaneous trans-hepatic biliary drainage due to acute calculous cholecystitis. The hypotension persisted for 3 hours despite massive hydration with vasoconstrictive drugs. However, skin manifestations were not present and the male patient had already suffered hypotension accompanied with fever and chills 2 hours before the study. We judged that the symptoms were not related to contrast media agent reaction and were more likely related with aggravation of preexisting sepsis after the procedure. In the other case, although the patient complained of urticaria after percutaneous nephrostomy change, he had preexisting urticaria for a week before the contrast media study. Those two reactions were determined not to be associated with ICM by an experienced allergist.
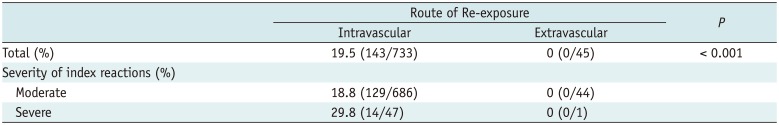
Finally, there was no case of recurrent AARs after extravascular administration of ICM in patients with a history of moderate to severe AARs (Table 2).
In a total of 257 patients with moderate (n = 235) or severe (n = 22) index AARs, a total of 733 radiologic examinations were subsequently performed with intravascular ICM administration. The number of intravascular re-exposures to ICM per person ranged from 1 to 16 (median, 2). The time interval between the index AAR and the first re-exposure to ICM via an intravascular route was from 2 to 1071 days (median, 291 days). All of the contrast agents were LOCMs (Table 1).
Of 257 patients, 170 with re-exposure did not have recurrent hypersensitivity reactions upon subsequent exposure to ICM. Eighty-seven patients (33.9%) had mild to severe degree of recurrent AARs when exposed to ICM intravascularly. On a case-by-case basis, 143 cases of recurrent AARs occurred among 733 cases of intravascular ICM re-exposure (19.5%), which comprised 67 mild, 58 moderate, and 18 severe recurrent AARs. In 235 patients with a moderate degree of index AARs, 129 AARs developed in 686 cases of intravascular ICM re-exposure (18.8%). They comprised 66 mild, 53 moderate, and 10 severe recurrent AARs. In 22 patients with severe degree of index AARs, 14 recurrent AARs developed in 47 cases of intravascular ICM re-exposure (29.8%). They comprised one mild, 5 moderate, and 8 severe recurrent AARs. There was a significant difference in the occurrence of recurrent AARs between the two routes with respect to the number of patients (p < 0.001) and events (p < 0.001), respectively.
Of the 13 patients exposed to ICM through both intravascular and extravascular routes, 5 patients experienced 9 recurrent AARs among 49 intravascular ICM re-exposures (18.4%). No patient experienced recurrent AARs with extravascular ICM administration.
Although almost all cases of AARs to ICM are associated with intravascular administration, AARs rarely occur after the administration of ICM into the body cavities including the GI and GU tracts (131112). When the ICM is administered for the opacification of the GI tract, it is normally absorbed in small amounts (approximately 1% to 2%) (161718). Since AARs are not considered to be dose-related and can occur with less than 1 mL of ICM, it is expected that AARs can occur even with the administration of ICM into body cavities. Appropriately, the European Society of Urogenital Radiology guidelines on contrast media recommend the same precautions as for intravascular ICM administration in cases of contrast media administration into body cavities (1). The necessity of screening and premedication during extravascular administration of contrast media has been advocated (317).
Few studies have investigated AARs to extravascular ICM administration (19). Therefore, some centers do not consider previous anaphylaxis to ICM as a contraindication to extravascular administration of ICM or the need to pre-medicate (1920212223). Presently, AARs after extravascular administration were absent in high-risk patients even without premedication, while the incidence of recurrent AARs upon intravascular administration was 19.5% in high-risk patients who had received premedication. It is possible that the mild AARs were missed due to lack of attention in the extravascular ICM administration and the retrospective nature of this study. However, we believe that a significant difference in the incidence of AAR between intravascular and extravascular administration could not be adequately explained in that manner. We think that there are two plausible explanations for our results. First, in spite of the conventional concept of dose dependency in AAR, we believe from our clinical experience that dose-dependency might be present in mild form of AARs. The absorption rate during extravascular administration would be slower than for intravascular administration. Shortly after administration, less absorption of extravascular ICM occurs compared to intravascular ICM (1719). The absorbed amount would be insufficient to reach a concentration that provokes AARs. In this manner, AARs can be hard to detect until the patient has left the hospital. Second, the different chemical properties between the re-exposed ICMs which were extravascularly administered and the culprit ICMs would explain the low rate of the recurrent AARs with extravascular ICM administration. Presently, most (86.7%) cases of extravascular ICM administration used HOCMs, while all culprit ICMs were LOCMs. Cross-reactivity is more pronounced among drugs of similar chemical structure (24). Based on this concept of cross-reactivity in drug allergy, there would be less cross-reactivity between HOCMs and LOCMs than between different LOCMs (925). We postulate that a synergic effect of small delayed absorption of ICM to systemic circulation and low cross-reactivity of HOCMs with LOCMs may have induced the absence of recurrent AARs on extravascular ICM administration.
Although the risk of a short course of steroid use for premedication is extremely low, precautions should be taken when steroids are used in patients with uncontrolled hypertension, diabetes, tuberculosis, systemic fungal infections, peptic ulcer disease, or diverticulitis (26). Furthermore, anaphylaxis to oral glucocorticoids have been rarely reported (327). Therefore, before deciding to premedicate an at risk patient, the risk of premedication must be weighed against the risk of AARs. In this context, our results may indicate that in cases of extravascular ICM administration, pre-medicating at risk patients in the similar manner before intravascular ICM administration could lead to over-premedicated cases.
Our study has several limitations. First, only relatively small number of patients underwent imaging studies with extravascular ICM administration compared to the number of patients with intravascular route administration. Second, because of the retrospective study design, a few cases of mild or delayed AARs might have been missed.
In conclusion, for high-risk patients with a history of moderate or severe reactions to ICM, AARs upon extravascular administration of ICM are significantly infrequent compared with intravascular ICM administration.
References
1. Thomsen HS, Morcos SK. Contrast media and the kidney: European Society of Urogenital Radiology (ESUR) guidelines. Br J Radiol. 2003; 76:513–518. PMID: 12893691.

2. Hunt CH, Hartman RP, Hesley GK. Frequency and severity of adverse effects of iodinated and gadolinium contrast materials: retrospective review of 456,930 doses. AJR Am J Roentgenol. 2009; 193:1124–1127. PMID: 19770337.

3. ACR Committee on Drugs and Contrast Media. ACR manual on contrast media. Ver 10.2. American College of Radiology;2016.
4. Cochran ST, Bomyea K, Sayre JW. Trends in adverse events after IV administration of contrast media. AJR Am J Roentgenol. 2001; 176:1385–1388. PMID: 11373197.

5. Brockow K, Christiansen C, Kanny G, Clément O, Barbaud A, Bircher A, et al. ENDA. EAACI Interest Group on Drug Hypersensitivity. Management of hypersensitivity reactions to iodinated contrast media. Allergy. 2005; 60:150–158. PMID: 15647034.

6. Park EA, Lee W, Kang DK, Kim SJ, Kim YJ, Kim Y, et al. Comparison of iohexol-380 and iohexol-350 for coronary CT angiography: a multicenter, randomized, double-blind phase 3 trial. Korean J Radiol. 2016; 17:330–338. PMID: 27134522.

7. Morcos SK. Acute serious and fatal reactions to contrast media: our current understanding. Br J Radiol. 2005; 78:686–693. PMID: 16046418.

8. Tramèr MR, von Elm E, Loubeyre P, Hauser C. Pharmacological prevention of serious anaphylactic reactions due to iodinated contrast media: systematic review. BMJ. 2006; 333:675. PMID: 16880193.

9. Lee SY, Yang MS, Choi YH, Park CM, Park HW, Cho SH, et al. Stratified premedication strategy for the prevention of contrast media hypersensitivity in high-risk patients. Ann Allergy Asthma Immunol. 2017; 118:339–344.e1. PMID: 28087383.

10. Yoon SH, Lee SY, Kang HR, Kim JY, Hahn S, Park CM, et al. Skin tests in patients with hypersensitivity reaction to iodinated contrast media: a meta-analysis. Allergy. 2015; 70:625–637. PMID: 25649510.

11. Miller SH. Anaphylactoid reaction after oral administration of diatrizoate meglumine and diatrizoate sodium solution. AJR Am J Roentgenol. 1997; 168:959–961. PMID: 9124149.

12. Gmeinwieser J, Erhardt W, Reimann HJ, Babic R, Speck U, Wenzel-Hora B. Side effects of water-soluble contrast agents in upper gastrointestinal tract. Invest Radiol. 1990; 25(Suppl 1):S27–S28. PMID: 2283247.

13. Pan JJ, Draganov PV. Adverse reactions to iodinated contrast media administered at the time of endoscopic retrograde cholangiopancreatography (ERCP). Inflamm Allergy Drug Targets. 2009; 8:17–20. PMID: 19275689.

14. Skucas J. Anaphylactoid reactions with gastrointestinal contrast media. AJR Am J Roentgenol. 1997; 168:962–964. PMID: 9124150.

15. Witten DM, Hirsch FD, Hartman GW. Acute reactions to urographic contrast medium: incidence, clinical characteristics and relationship to history of hypersensitivity states. Am J Roentgenol Radium Ther Nucl Med. 1973; 119:832–840.
16. Pirmohamed M, Naisbitt DJ, Gordon F, Park BK. The danger hypothesis--potential role in idiosyncratic drug reactions. Toxicology. 2002; 181-182:55–63. PMID: 12505285.

17. Draganov P, Cotton PB. Iodinated contrast sensitivity in ERCP. Am J Gastroenterol. 2000; 95:1398–1401. PMID: 10894570.

18. Mann K, Rendl J, Busley R, Saller B, Seybold S, Hoermann R, et al. Systemic iodine absorption during endoscopic application of radiographic contrast agents for endoscopic retrograde cholangiopancreaticography. Eur J Endocrinol. 1994; 130:498–501. PMID: 8180679.

19. Davis PL. Anaphylactoid reactions to the nonvascular administration of water-soluble iodinated contrast media. AJR Am J Roentgenol. 2015; 204:1140–1145. PMID: 26001221.

20. Park HJ, Park JW, Yang MS, Kim MY, Kim SH, Jang GC, et al. Re-exposure to low osmolar iodinated contrast media in patients with prior moderate-to-severe hypersensitivity reactions: a multicentre retrospective cohort study. Eur Radiol. 2017; 27:2886–2893. PMID: 27975150.

21. Davenport MS, Cohan RH, Caoili EM, Ellis JH. Repeat contrast medium reactions in premedicated patients: frequency and severity. Radiology. 2009; 253:372–379. PMID: 19789241.

22. Shehadi WH. Contrast media adverse reactions: occurrence, recurrence, and distribution patterns. Radiology. 1982; 143:11–17. PMID: 7063711.

23. Freed KS, Leder RA, Alexander C, DeLong DM, Kliewer MA. Breakthrough adverse reactions to low-osmolar contrast media after steroid premedication. AJR Am J Roentgenol. 2001; 176:1389–1392. PMID: 11373198.

24. Brockow K, Romano A, Aberer W, Bircher AJ, Barbaud A, Bonadonna P, et al. European Network of Drug Allergy and the EAACI Interest Group on Drug Hypersensitivity. Skin testing in patients with hypersensitivity reactions to iodinated contrast media - a European multicenter study. Allergy. 2009; 64:234–241. PMID: 19178403.

25. Sancak S, Jaeschke H, Eren F, Tarcin O, Guellueoglu B, Sen LS, et al. High prevalence of TSHR/Gsα mutation-negative clonal hot thyroid nodules (HNs) in a Turkish cohort. Horm Metab Res. 2011; 43:562–568. PMID: 21773967.

26. Lasser EC, Berry CC, Mishkin MM, Williamson B, Zheutlin N, Silverman JM. Pretreatment with corticosteroids to prevent adverse reactions to nonionic contrast media. AJR Am J Roentgenol. 1994; 162:523–526. PMID: 8109489.

27. Wolf GL, Mishkin MM, Roux SG, Halpern EF, Gottlieb J, Zimmerman J, et al. Comparison of the rates of adverse drug reactions. Ionic contrast agents, ionic agents combined with steroids, and nonionic agents. Invest Radiol. 1991; 26:404–410. PMID: 2055736.
Table 1
Demographics of Patients with Intravascular or Extravascular Re-exposure to Iodinated Contrast Media
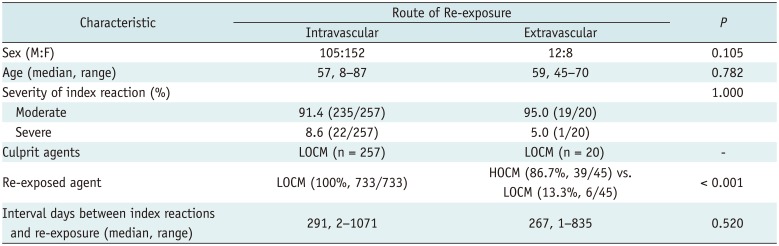
Table 2
Percentage of Recurrent Acute Allergic-Like Reactions Based on Re-exposure Route of Iodinated Contrast Media
| Route of Re-exposure | P | ||
|---|---|---|---|
| Intravascular | Extravascular | ||
| Total (%) | 19.5 (143/733) | 0 (0/45) | < 0.001 |
| Severity of index reactions (%) | |||
| Moderate | 18.8 (129/686) | 0 (0/44) | |
| Severe | 29.8 (14/47) | 0 (0/1) | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download