Abstract
Although the causes of hypertension are usually unknown, about 10% of the cases occur secondary to specific etiologies, which are often treatable. Common categories of secondary hypertension include renal parenchymal disease, renovascular stenosis, vascular and endocrinologic disorders. For diseases involving the renal parenchyma and adrenal glands, ultrasonography (US), computed tomography (CT) or magnetic resonance (MR) imaging is recommended. For renovascular stenosis and vascular disorders, Doppler US, conventional or noninvasive (CT or MR) angiography is an appropriate modality. Nuclear imaging can be useful in the differential diagnosis of endocrine causes. Radiologists should understand the role of each imaging modality and its typical findings in various causes of secondary hypertension. This article focuses on appropriate imaging approaches in accordance with the categorized etiologies leading to hypertension.
Secondary hypertension is the term embracing hypertension related to a specific etiology. It accounts for about 10% of all hypertensive patients (12). It should be suspected in cases of new or sudden onset of hypertension in patients before the third or after the sixth decade of life, persistent hypertension despite medication, or patients with typical signs and symptoms indicating specific disorders (3). Common etiologies of secondary hypertension can be categorized in accordance with the anatomy or physiology as follows: renal parenchymal diseases, renovascular stenosis, other vascular diseases, endocrinologic disturbances, diseases of the central nervous system, obstructive sleep apnea, and drug- or diet-related conditions (4).
Secondary hypertension is clinically important because identifying its etiology and treating the condition can effectively lower the blood pressure (56). However, routine screening for secondary hypertension is neither essential nor cost-effective due to its low prevalence. Radiologists should understand the role of each imaging modality and be familiar with the appropriate selection of diagnostic tests and their findings to help the referring physicians in prompt identification of patients with treatable causes. Therefore, in this article, we present the roles of multiple imaging modalities and typical imaging findings indicating a specific etiology of secondary hypertension. We also present a diagnostic algorithm consisting of patients' symptoms, laboratory findings and appropriate imaging modalities, depending on the specific condition of secondary hypertension.
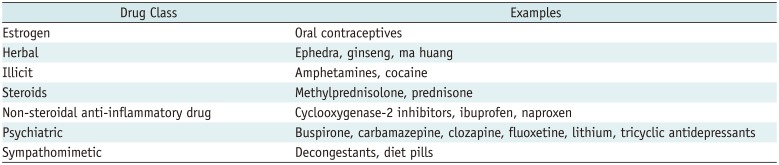
Once the patient is identified as being hypertensive, detailed history taking and physical examination should be performed to determine if it is related to a specific cause. Some clinical symptoms and signs may provide clues. Laboratory findings and multimodality imaging further discriminate specific causes. Figure 1 illustrates a flow chart consisting of patients' symptoms, laboratory findings, and appropriate imaging modalities depending on each specific condition of secondary hypertension. Before conducting diagnostic tests, keeping the pros and cons of each diagnostic modality in mind can prevent patients from undergoing unnecessary tests and thereby protect them from potential harm (Table 1). Some medications may have hypertensive effects, and therefore, the possibility of drug-induced hypertension should be excluded during the initial history taking (Table 2) (4).
This entity consists of up to 80% of all categories of secondary hypertension (78), and accounts for 2–5% of all causes of hypertension. A vicious cycle is formed where chronic kidney disease (CKD) and hypertension aggravate each other (9). Thus, early application of antihypertensive agents intervening in the renin-angiotensin-aldosterone (RAA) system, such as angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, is important (10). Renal parenchymal hypertension is suspected when a hypertensive patient shows proteinuria, hematuria, elevated blood urea nitrogen and creatinine, and decreased glomerular filtration rate. Renal ultrasonography (US) or computed tomography (CT) is considered for morphological evaluation of the kidneys (4). Diabetic nephropathy, glomerulonephritis, nephrosclerosis and polycystic kidney disease (PKD) are common causes in this subgroup.
Three factors are known to contribute to hypertension in chronic glomerulonephritis: 1) sodium and water retention, 2) excessive activity of the RAA system, and 3) increased sympathetic tone (1112). Therefore, diuretics, calcium channel blockers or RAA system inhibitors are indicated for the treatment of this entity. Renal transplantation can be considered in refractory cases.
Ultrasonography demonstrates increased parenchymal echogenicity compared to that of the liver and spleen, with a small kidney, defined as the one less than 9 cm in length, or the one smaller than its counterpart with discrepancy greater than 1.5 cm (13). CT depicts cortical thinning and irregularities in the kidneys with atrophic changes (Fig. 2).
Polycystic kidney disease is inherited either in an autosomal dominant or recessive manner, with the former being much more common (14). It is characterized by progressive development of numerous renal cysts with gradual decline in renal function. Nearly all patients meeting the criteria for CKD and 60% of patients with preserved renal function develop hypertension (1516). US and CT show well-defined, thin-walled bilateral renal cysts, along with cysts in other organs such as liver, ovary, spleen, seminal vesicles, prostate and pancreas depending on the type. In patients with a history of prolonged dialysis, increased incidence of renal cell carcinoma is reported, and thus, their renal cysts may be followed-up on the basis of Bosniak classification (17) (Supplementary Table 1 in the online-only Data Supplement).
About 10–20% of the patients with autosomal dominant PKD have accompanying intracranial aneurysms (18). Therefore, brain CT or magnetic resonance (MR) angiography is indicated for evaluation of the presence and size of a cerebral aneurysm in patients with a family history of hemorrhagic stroke (Fig. 3) (1920).
Steno-occlusion of the renal artery accounts for about 1% of all hypertensive cases (21) and it affects 15–30% of patients with renovascular disease (22). Renovascular hypertension is mediated by renin and occurs in response to renal ischemia. Physical examination may detect an abdominal bruit, and imaging modalities would demonstrate kidney size discrepancy.
The most common etiology differs according to the age group: atherosclerosis in the elderly, and fibromuscular dysplasia (FMD) in the young group. An atherosclerotic change is characterized by progressive luminal stenosis mainly involving the renal arteries, along with other arteries because of its systemic nature. When it affects the renal arteries, it typically involves the proximal segment, within 2 cm from the ostia (Fig. 4) (22). On the other hand, FMD affects the middle or distal segment of the renal arteries. It is an idiopathic vascular disorder with a predilection for renal arteries in young adults, and it occurs more often in females than in males (23). The characteristic “string of beads” appearance of the affected artery represents alternating segments of thin and thick portions of the collagen-containing medial layer, and it is observed in the medial dysplasia type (Fig. 5). Other types such as intimal fibroplasia or adventitial fibroplasia may appear as smooth, concentric stenosis or even aneurysm. Doppler US may reveal a decreased flow with the pulsus tardus et parvus wave pattern.
Although CT or MR angiography effectively assesses renovascular stenosis, conventional angiography is occasionally necessary for obtaining higher resolution in validating branch vessels (24). Conventional angiography is also essential when a therapeutic option, such as transluminal angioplasty, is considered (Fig. 4). Caution is advised when patients with compromised renal function undergo contrast-enhanced imaging, as iodine or gadolinium based contrast media is a known risk factor for contrast-induced nephropathy and nephrogenic systemic fibrosis, respectively (2526). For these patients, MR angiography without contrast material or Doppler US can be an alternative.
Diseases affecting blood vessels other than renal arteries can also manifest as hypertension. Any condition causing steno-occlusion of the aorta is included in this entity, such as coarctation of the aorta (COA), aortitis, or systemic diseases that may affect the blood vessels (4). Common clinical vignettes include audible bruits over the neck, chest, or abdomen, and difference in blood pressure between the upper and lower extremities (27).
Coarctation of the aorta is a constrictive congenital anomaly of the aorta and the second most common cause of hypertension in children and young adults (28). The incidence of hypertension increases rapidly as patients get older, and it is reported to be 1.36% in the pediatric age group and 28.9% and 45.7% in the age groups of 20 to 29 and 30 to 39 years, respectively (29). It is classified into the preductal and postductal types, according to the location of the coarctation in relation to the ductus arteriosus (3031). Other frequently associated cardiovascular anomalies are bicuspid aortic valve, ventricular septal defect, mitral valve anomalies, and even cerebral aneurysm (32). It is known that 15% of Turner syndrome patients present with COA (33). Clues on a chest radiograph include left ventricular hypertrophy, small aortic arch contour and/or notching of the ribs. CT or MR demonstrates location of the stenotic portion, various collaterals, and other associated anomalies (Fig. 6). Early detection and surgical correction are warranted to achieve a better prognosis. If left unrepaired, the mortality reaches as high as 90% at age 50 (34).
The mid-aortic dysplastic syndrome is a type of COA, which is characterized by segmental narrowing of the distal thoracic or abdominal aorta, or both, either due to a congenital or an acquired cause (35). Incomplete fusion or overfusion of embryonic dorsal aortas during fourth week of gestation is thought to be a congenital cause, whereas Takayasu arteritis, neurofibromatosis (NF), FMD, retroperitoneal fibrosis are common causes in acquired cases (3637). Renal artery involvement is common in these patients, and the main cause of death is the cardiovascular complication arising from progressive hypertension (36). CT, MR or conventional angiography shows characteristic segmental narrowing of the aortic mid-portion with involvement of visceral branches such as the renal or superior mesenteric artery. Depending on chronicity, various collaterals may develop via internal mammary, epigastric and circumflex iliac arteries (Fig. 7). Characteristic CT findings in acquired cases include vascular calcification and/or concentric vascular wall enhancement (38).
The endocrinologic etiologies comprise about 3% of secondary hypertension cases. They are characterized by excessive hormone secretion, and in many cases, they are correctable unless the diagnosis is made after end organ damage occurs. Screening tests targeting excessive hormones have a relatively higher diagnostic performance than those targeting other causes of secondary hypertension (39).
Primary aldosteronism is characterized by excessive aldosterone secretion from adrenal adenoma or bilateral adrenal hyperplasia. Patients present with hypertension and hypokalemic metabolic alkalosis with an absence of a history of diuretic therapy (4041). Plasma aldosterone/renin ratio ≥ 20 sensitively indicates this condition (42).
When lipid component is evident within the adrenal mass on precontrast CT or chemical shift MR imaging, benign lesions including adenomas are readily diagnosed (4344). However, when an adrenal incidentaloma is lipid-poor, multiphasic contrast-enhanced CT is usually required. The following formulae named absolute percentage washout (APW) and relative percentage washout (RPW) are utilized for characterization of an adrenal incidentaloma (45):
APW = 100 × (venous attenuation − delayed attenuation) / (venous attenuation − precontrast attenuation)
RPW = 100 × (venous attenuation − delayed attenuation) / venous attenuation
Venous attenuation refers to the CT number obtained in the portal venous phase, usually acquired within 70–90 seconds after contrast administration. Delayed attenuation is obtained from the time period varying between 5 and 15 minutes after contrast injection depending on the researcher's need (4647). The diagnostic accuracy of APW greater than 60 and RPW greater than 40 for diagnosing adrenal adenoma was found to be 94.2% for lipid-poor adrenal incidentaloma (48). When MR and CT are inconclusive, radionuclide studies and/or adrenal venous sampling can be considered (Fig. 8) (49) .
Cushing's syndrome is characterized by autonomous and excessive secretion of cortisol. It should be considered when a hypertensive patient presents with central obesity, moon face, buffalo hump, or red striae. Less specific features include diabetes mellitus, dyslipidemia, osteoporosis, and urolithiasis, with laboratory clues being eosinopenia and hypokalemia (39).
Excluding exogenous corticosteroid use, which is the most common cause of Cushing's syndrome, pathology can be localized anywhere in the hypothalamic-pituitary-adrenal axis (50). Pituitary MR imaging is indicated in patients with adrenocorticotropic hormone (ACTH)-dependent type of Cushing's syndrome since corticotroph adenoma of the pituitary gland comprises most of the cases (80–85%) (51). Adrenal CT should be considered to detect incidentaloma in ACTH-dependent type of Cushing's syndrome without pituitary pathology, or in ACTH-independent type (52). Patients with excessive ACTH secretion may demonstrate bilateral adrenal cortical hyperplasia with strong enhancement on CT and hypermetabolism in bilateral adrenal cortices on whole body positron emission tomography (PET) (Fig. 9).
Pheochromocytomas are rare tumors originating from the adrenal medulla. When they occur in the extra-adrenal paraganglia, they are referred to as paragangliomas. Due to excess of catecholamines and their metabolites, episodic headaches, palpitations, sweating and pallor develop frequently (53). The traditional “10% rule” has been known to be applicable to their extra-adrenal locations, the rate of bilaterality, multiplicity, and malignancy (54). In familial cases, they are often associated with multiple endocrine neoplasia type II, von Hippel-Lindau syndrome, and NF type I (53). Laboratory tests are performed by measuring the metanephrine level in a 24-hour urine or plasma sample.
Pheochromocytomas can have a varied appearance on CT due to variable contents of fat, hemorrhage and/or calcifications, but the solid components are usually well enhanced. Typical MR findings include T1-hypo- and T2-hyperintensities. Nuclear imaging tests such as 123I-metaiodobenzylguanidine scintigraphy or PET may show high diagnostic accuracy in false-negative cases on CT or MR (Fig. 10) (55).
Either hyper- or hypothyroidism can cause systemic or pulmonary hypertension. Patients with hyperthyroidism usually manifest with systolic hypertension, via upregulation of catecholamine action and β-adrenergic receptors (56), while those with hypothyroidism show elevated diastolic blood pressure, with exaggerated sympathetic tone and α-adrenergic response (57).
Ultrasonography is the most common imaging modality for thyroid assessment. Although thyroid dysfunction does not necessarily accompany abnormal US findings, certain etiologies of hyper- or hypothyroidism are associated with diffuse thyroid diseases or thyroiditis. In these cases, thyroid can show diffuse enlargement and heterogeneous echogenicity, with variable vascularity (Fig. 11). Nuclear scintigraphy with various radiotracers (99mTc, 131I or 123I) can be useful in the evaluation of thyroid function and metabolic activity (58).
Hyperparathyroidism can be classified into primary and secondary forms. It results from either a pathologic condition of the parathyroid gland itself (adenoma, most commonly), or compensatory hyperplasia following other systemic conditions, such as CKD, vitamin D deficiency, and malabsorption in the gastrointestinal tract (59). It is estimated that 40–65% of patients with hyperparathyroidism develop hypertension (60). Since the parathyroid hormone regulates calcium metabolism, patients may manifest with nephrolithiasis, hypercalciuria, and overt skeletal diseases.
When a nodule exceeding 1 cm in size is found in an extrathyroid location on US or CT, parathyroid adenoma or hyperplasia can be suspected (61). Sestamibi scan is commonly used because it is taken up more avidly and retained longer by adenomatous and hyperplastic parathyroid than by the thyroid tissue. Therefore, characteristics of hyperfunctioning parathyroid tissue are clearly noted on delayed images, approximately 2 hours after Sestamibi administration (61). Plain radiographs can be helpful in assessing the skeletal manifestation, including subperiosteal resorption, rugger-jersey spine, and brown tumors. Parathyroid adenomas are hypoechoic compared to the overlying thyroid gland on gray-scale US due to relative hypercellularity. Multiphase CT may be useful for detection of the adenoma; a precontrast scan may discriminate hypodense adenoma from iodine-rich thyroid gland, while an enhanced scan may show a hypervascular nature. Larger lesions may show heterogeneous attenuation or calcifications (Fig. 12) (62).
Common causes of secondary hypertension include renal parenchymal diseases, renovascular stenosis, vascular diseases, and endocrinologic disturbances. Although some clinical vignettes strongly indicate secondary hypertension, imaging workup is essential for the differential diagnosis as symptoms are often nonspecific. US can be the initial modality of choice for renal parenchymal disease, renovascular hypertension, thyroid and parathyroid dysfunctions. CT is widely used for evaluation of renal parenchyma, adrenal incidentalomas, thyroid or parathyroid nodules, as well as for vascular assessment in cases of vascular or renovascular diseases. MR can be an alternative in patients who have difficulty in undergoing CT. MR angiography without contrast media can be recommended for patients with renal dysfunction. Conventional angiography can be used not only for diagnosis but also for therapeutic revascularization in patients with vascular or renovascular hypertension. Nuclear scan with various radiotracers can provide further information on the function of endocrine organs. Radiologist should be familiar with the role of each imaging modality and the characteristic findings of each cause of secondary hypertension, and they should select.
References
1. Sinclair AM, Isles CG, Brown I, Cameron H, Murray GD, Robertson JW. Secondary hypertension in a blood pressure clinic. Arch Intern Med. 1987; 147:1289–1293. PMID: 3606286.

2. Anderson GH Jr, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens. 1994; 12:609–615. PMID: 7930562.

3. Rimoldi SF, Scherrer U, Messerli FH. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J. 2014; 35:1245–1254. PMID: 24366917.

4. Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, et al. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014; 37:253–390. PMID: 24705419.

5. Akpunonu BE, Mulrow PJ, Hoffman EA. Secondary hypertension: evaluation and treatment. Dis Mon. 1996; 42:609–722. PMID: 8948319.

6. Chiong JR, Aronow WS, Khan IA, Nair CK, Vijayaraghavan K, Dart RA, et al. Secondary hypertension: current diagnosis and treatment. Int J Cardiol. 2008; 124:6–21. PMID: 17462751.

7. Ferguson RK. Cost and yield of the hypertensive evaluation. Experience of a community-based referral clinic. Ann Intern Med. 1975; 82:761–776. PMID: 1138586.
8. Danielson M, Dammström B. The prevalence of secondary and curable hypertension. Acta Med Scand. 1981; 209:451–455. PMID: 7257863.

9. Kimura G. Clinical pathology and treatment of renin-angiotensin system 2. Chronic kidney disease and the renin-angiotensin system. Intern Med. 2007; 46:1295–1298. PMID: 17704608.

10. Usami T, Nakao N, Fukuda M, Takeuchi O, Kamiya Y, Yoshida A, et al. Maps of end-stage renal disease and amounts of angiotensin-converting enzyme inhibitors prescribed in Japan. Kidney Int. 2003; 64:1445–1449. PMID: 12969164.

11. Ihm CG. Hypertension in chronic glomerulonephritis. Electrolyte Blood Press. 2015; 13:41–45. PMID: 26848302.

12. Joles JA, Koomans HA. Causes and consequences of increased sympathetic activity in renal disease. Hypertension. 2004; 43:699–706. PMID: 14981063.

13. Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA task force on practice guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006; 113:e463–e654. PMID: 16549646.

14. Nahm AM, Henriquez DE, Ritz E. Renal cystic disease (ADPKD and ARPKD). Nephrol Dial Transplant. 2002; 17:311–314. PMID: 11812890.

15. Higashihara E, Aso Y, Shimazaki J, Ito H, Koiso K, Sakai O. Clinical aspects of polycystic kidney disease. J Urol. 1992; 147:329–332. PMID: 1732586.

16. Kelleher CL, McFann KK, Johnson AM, Schrier RW. Characteristics of hypertension in young adults with autosomal dominant polycystic kidney disease compared with the general U.S population. Am J Hypertens. 2004; 17(11 Pt 1):1029–1034. PMID: 15533729.

17. Wood CG 3rd, Stromberg LJ 3rd, Harmath CB, Horowitz JM, Feng C, Hammond NA, et al. CT and MR imaging for evaluation of cystic renal lesions and diseases. Radiographics. 2015; 35:125–141. PMID: 25590393.

18. Pirson Y, Chauveau D, Torres V. Management of cerebral aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2002; 13:269–276. PMID: 11752048.

19. Ring T, Spiegelhalter D. Risk of intracranial aneurysm bleeding in autosomal-dominant polycystic kidney disease. Kidney Int. 2007; 72:1400–1402. PMID: 17882153.

21. Mann SJ, Pickering TG. Detection of renovascular hypertension. State of the art: 1992. Ann Intern Med. 1992; 117:845–853. PMID: 1416561.
22. Soulez G, Oliva VL, Turpin S, Lambert R, Nicolet V, Therasse E. Imaging of renovascular hypertension: respective values of renal scintigraphy, renal Doppler US, and MR angiography. Radiographics. 2000; 20:1355–1368. discussion 1368-1372. PMID: 10992024.

24. Olin JW, Sealove BA. Diagnosis, management, and future developments of fibromuscular dysplasia. J Vasc Surg. 2011; 53:826–836.e1. PMID: 21236620.

25. Mohammed NM, Mahfouz A, Achkar K, Rafie IM, Hajar R. Contrast-induced nephropathy. Heart Views. 2013; 14:106–116. PMID: 24696755.

26. Hellman RN. Gadolinium-induced nephrogenic systemic fibrosis. Semin Nephrol. 2011; 31:310–316. PMID: 21784280.

27. Kim HD, Kim MN, Kim SA, Choi SI, Choi JY, Seo JH, et al. Resistant hypertension caused by stenosis of the aorta in elderly women: three case reports. Clin Hypertens. 2014; 20:5. PMID: 26893910.

28. Viera AJ, Neutze DM. Diagnosis of secondary hypertension: an age-based approach. Am Fam Physician. 2010; 82:1471–1478. PMID: 21166367.
29. Wu MH, Chen HC, Kao FY, Huang SK. Risk of systemic hypertension and cerebrovascular accident in patients with aortic coarctation aged <60 years (from a national database study). Am J Cardiol. 2015; 116:779–784. PMID: 26100586.
30. Abbruzzese PA, Aidala E. Aortic coarctation: an overview. J Cardiovasc Med (Hagerstown). 2007; 8:123–128. PMID: 17299295.

31. Nance JW, Ringel RE, Fishman EK. Coarctation of the aorta in adolescents and adults: a review of clinical features and CT imaging. J Cardiovasc Comput Tomogr. 2016; 10:1–12. PMID: 26639936.

32. Sebastià C, Quiroga S, Boyé R, Perez-Lafuente M, Castellà E, Alvarez-Castells A. Aortic stenosis: spectrum of diseases depicted at multisection CT. Radiographics. 2003; 23(Spec No):S79–S91. PMID: 14557504.

33. Ho VB, Bakalov VK, Cooley M, Van PL, Hood MN, Burklow TR, et al. Major vascular anomalies in Turner syndrome: prevalence and magnetic resonance angiographic features. Circulation. 2004; 110:1694–1700. PMID: 15353492.

34. Campbell M. Natural history of coarctation of the aorta. Br Heart J. 1970; 32:633–640. PMID: 5470045.

35. Connolly JE, Wilson SE, Lawrence PL, Fujitani RM. Middle aortic syndrome: distal thoracic and abdominal coarctation, a disorder with multiple etiologies. J Am Coll Surg. 2002; 194:774–781. PMID: 12081068.
36. Delis KT, Gloviczki P. Middle aortic syndrome: from presentation to contemporary open surgical and endovascular treatment. Perspect Vasc Surg Endovasc Ther. 2005; 17:187–203. PMID: 16273154.

37. Daghero F, Bueno N, Peirone A, Ochoa J, Torres GF, Ganame J. Coarctation of the abdominal aorta: an uncommon cause of arterial hypertension and stroke. Circ Cardiovasc Imaging. 2008; 1:e4. e6. PMID: 19808505.
39. Sica DA. Endocrine causes of secondary hypertension. J Clin Hypertens (Greenwich). 2008; 10:534–540. PMID: 18607139.

40. Gallay BJ, Ahmad S, Xu L, Toivola B, Davidson RC. Screening for primary aldosteronism without discontinuing hypertensive medications: plasma aldosterone-renin ratio. Am J Kidney Dis. 2001; 37:699–705. PMID: 11273868.

41. Eide IK, Torjesen PA, Drolsum A, Babovic A, Lilledahl NP. Low-renin status in therapy-resistant hypertension: a clue to efficient treatment. J Hypertens. 2004; 22:2217–2226. PMID: 15480108.
42. Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008; 93:3266–3281. PMID: 18552288.

43. Boland GW, Lee MJ, Gazelle GS, Halpern EF, McNicholas MM, Mueller PR. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998; 171:201–204. PMID: 9648789.

44. Sohaib SA, Peppercorn PD, Allan C, Monson JP, Grossman AB, Besser GM, et al. Primary hyperaldosteronism (Conn syndrome): MR imaging findings. Radiology. 2000; 214:527–531. PMID: 10671606.

45. Blake MA, Kalra MK, Sweeney AT, Lucey BC, Maher MM, Sahani DV, et al. Distinguishing benign from malignant adrenal masses: multi-detector row CT protocol with 10-minute delay. Radiology. 2006; 238:578–585. PMID: 16371582.

46. Korobkin M, Brodeur FJ, Francis IR, Quint LE, Dunnick NR, Londy F. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998; 170:747–752. PMID: 9490968.

47. Peña CS, Boland GW, Hahn PF, Lee MJ, Mueller PR. Characterization of indeterminate (lipid-poor) adrenal masses: use of washout characteristics at contrast-enhanced CT. Radiology. 2000; 217:798–802. PMID: 11110946.

48. Seo JM, Park BK, Park SY, Kim CK. Characterization of lipid-poor adrenal adenoma: chemical-shift MRI and washout CT. AJR Am J Roentgenol. 2014; 202:1043–1450. PMID: 24758658.

49. Rossi GP, Sacchetto A, Chiesura-Corona M, De Toni R, Gallina M, Feltrin GP, et al. Identification of the etiology of primary aldosteronism with adrenal vein sampling in patients with equivocal computed tomography and magnetic resonance findings: results in 104 consecutive cases. J Clin Endocrinol Metab. 2001; 86:1083–1090. PMID: 11238490.

50. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing's syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2014; 4:739–769. PMID: 24715566.

51. Sohaib SA, Hanson JA, Newell-Price JD, Trainer PJ, Monson JP, Grossman AB, et al. CT appearance of the adrenal glands in adrenocorticotrophic hormone-dependent Cushing's syndrome. AJR Am J Roentgenol. 1999; 172:997–1002. PMID: 10587135.

52. Rockall AG, Babar SA, Sohaib SA, Isidori AM, Diaz-Cano S, Monson JP, et al. CT and MR imaging of the adrenal glands in ACTH-independent cushing syndrome. Radiographics. 2004; 24:435–452. PMID: 15026592.

53. Sukor N. Endocrine hypertension--current understanding and comprehensive management review. Eur J Intern Med. 2011; 22:433–440. PMID: 21925049.
54. Tischler AS. Pheochromocytoma and extra-adrenal paraganglioma: updates. Arch Pathol Lab Med. 2008; 132:1272–1284. PMID: 18684026.

55. Leung K, Stamm M, Raja A, Low G. Pheochromocytoma: the range of appearances on ultrasound, CT, MRI, and functional imaging. AJR Am J Roentgenol. 2013; 200:370–378. PMID: 23345359.

56. Prisant LM, Gujral JS, Mulloy AL. Hyperthyroidism: a secondary cause of isolated systolic hypertension. J Clin Hypertens (Greenwich). 2006; 8:596–599. PMID: 16896276.

57. Marcisz C, Jonderko G, Kucharz EJ. Influence of short-time application of a low sodium diet on blood pressure in patients with hyperthyroidism or hypothyroidism during therapy. Am J Hypertens. 2001; 14:995–1002. PMID: 11710792.

58. Nachiappan AC, Metwalli ZA, Hailey BS, Patel RA, Ostrowski ML, Wynne DM. The thyroid: review of imaging features and biopsy techniques with radiologic-pathologic correlation. Radiographics. 2014; 34:276–293. PMID: 24617678.

59. Silverberg SJ, Bilezikian JP. The diagnosis and management of asymptomatic primary hyperparathyroidism. Nat Clin Pract Endocrinol Metab. 2006; 2:494–503. PMID: 16957763.

60. Feldstein CA, Akopian M, Pietrobelli D, Olivieri A, Garrido D. Long-term effects of parathyroidectomy on hypertension prevalence and circadian blood pressure profile in primary hyperparathyroidism. Clin Exp Hypertens. 2010; 32:154–158. PMID: 20504122.

61. Johnson NA, Tublin ME, Ogilvie JB. Parathyroid imaging: technique and role in the preoperative evaluation of primary hyperparathyroidism. AJR Am J Roentgenol. 2007; 188:1706–1715. PMID: 17515397.

62. Chandramohan A, Sathyakumar K, John RA, Manipadam MT, Abraham D, Paul TV, et al. Atypical ultrasound features of parathyroid tumours may bear a relationship to their clinical and biochemical presentation. Insights Imaging. 2014; 5:103–111. PMID: 24293304.

Supplementary materials
The online-only Data Supplement is available with this article at https://doi.org/10.3348/kjr.2018.19.2.272.
Fig. 1
Appropriate diagnostic imaging approaches consisting of patients' symptoms, laboratory findings, and imaging modalities depending on etiologies of secondary hypertension.
BP = blood pressure, CT = computed tomography, GFR = glomerular filtration rate, MR = magnetic resonance, US = ultrasonography,é = elevated, é = decreased
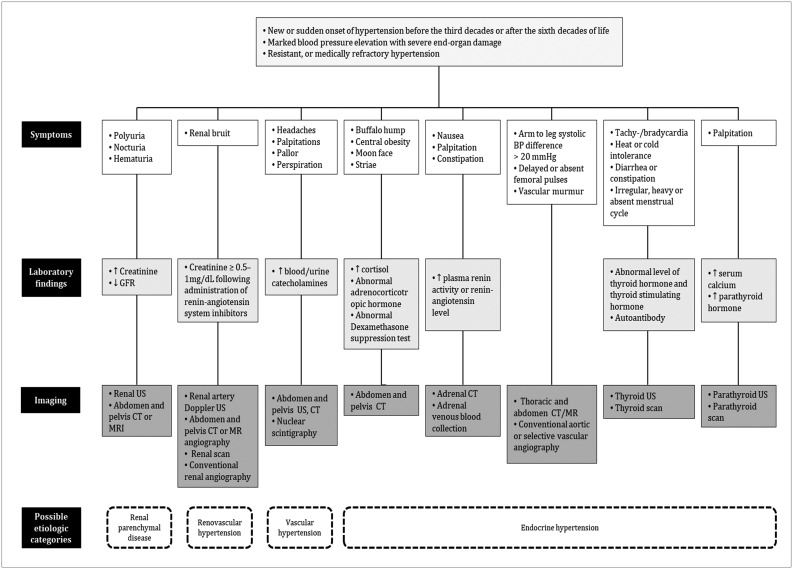
Fig. 2
Glomerulonephritis in 37-year-old male with long history of hypertension and nephrotic range proteinuria.
Renal US (A) demonstrates small kidney less than 9 cm in length, with relatively higher parenchymal echogenicity (arrowheads) compared to liver (arrow). CT images (B) from arterial (upper row) and delayed phases (lower row) show irregular contour with atrophic changes in bilateral kidneys (arrowheads). Aortic dissection (arrows), possible complication of long-standing hypertension, is also noted.
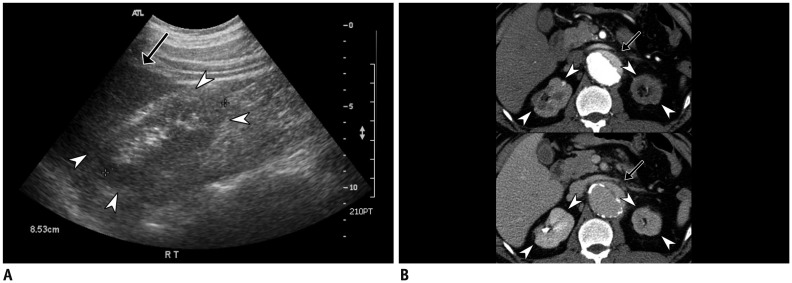
Fig. 3
Autosomal dominant polycystic kidney disease in 37-year-old male with hypertension and family history of hemorrhagic stroke.
Coronal CT image (A) shows multiple cysts of variable sizes in bilateral kidneys and liver. Volume-rendered image of left internal carotid artery (B) shows tiny aneurysm (arrowhead) at level of bifurcation.
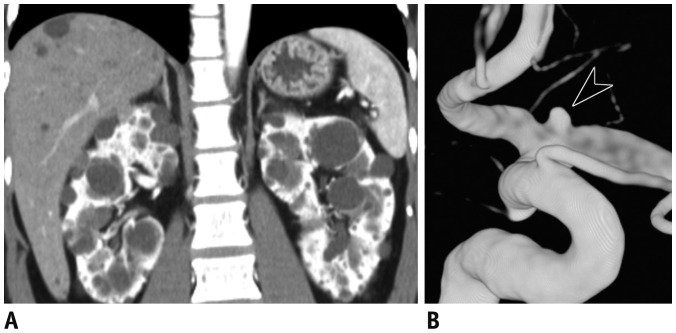
Fig. 4
Atherosclerotic renovascular stenosis in 76-year-old female with hypertension and recent onset azotemia.
Coronal CT image with maximum intensity projection (A) shows diffuse atherosclerotic change in aorta and its branches, with focal narrowing (arrow) of proximal segment of left renal artery. Conventional angiography (B) redemonstrated severe stenosis of left renal artery (arrowhead). Lesion was targeted by transluminal angioplasty (arrow), with resultant restoration of luminal diameter. Although blood pressure did not reduce immediately, azotemia was improved.
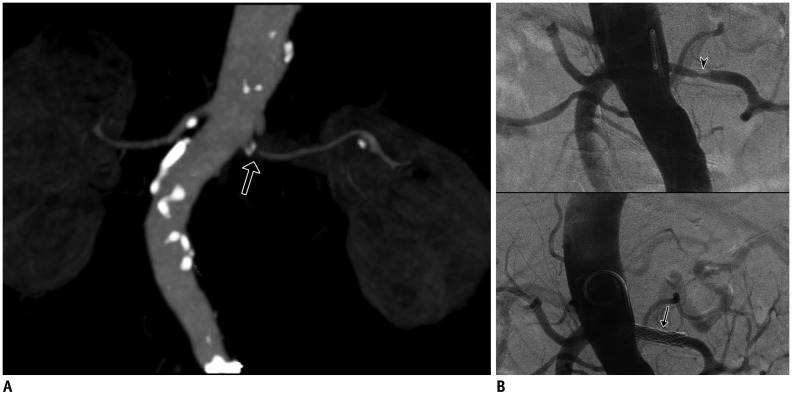
Fig. 5
Fibromuscular dysplasia in 20-year-old male with hypertension.
Maximum intensity projection image (A) shows focal narrowing with luminal irregularities in middle segment of right renal artery (arrows). Doppler US (B) reveals tardus-et-parvus waveform with low resistive index of right kidney (labeled as R) compared to left kidney (labeled as L). Aortography and selective right renal arteriography (C) confirmed focal stenosis with luminal irregularities (arrows) in right renal artery causing ipsilateral perfusion delay (arrowheads).
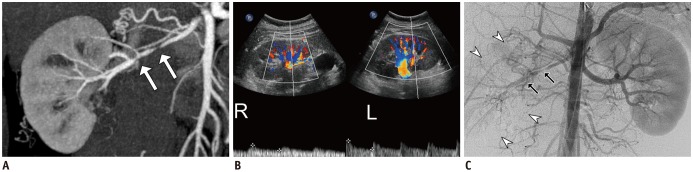
Fig. 6
Coarctation of aorta in 46-year-old female with hypertension and dyspnea on exertion.
Plain radiograph of chest (A) shows left ventricular hypertrophy, small contour of aortic arch (arrowheads), and mild notching of a few ribs (arrows). Volume-rendered CT image (B) reveals postductal type of aortic coarctation (arrowheads), with well-developed collaterals via internal mammary and intercostal arteries (arrows). Reformatted image of valve (C) shows bicuspid aortic valve (arrows), one of well-known anomalies associated with coarctation of aorta. Patient underwent corrective surgery (Bentall operation) with subsequent normalization of blood pressure.
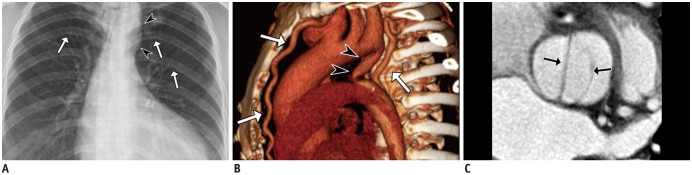
Fig. 7
Mid-aortic dysplastic syndrome in 62-year-old female with hypertension and intermittent claudication.
Serial axial CT image (A) shows segmental luminal narrowing of abdominal aorta with calcified vascular wall (arrowheads). Volume-rendered image (B) demonstrates aortic stenosis involving ostia of bilateral renal arteries (arrows). Maximum intensity projection in CT angiography (C) shows segmental narrowing of infrarenal abdominal aorta with concentric calcification (arrowheads), probably due to sequelae of Takayasu aortitis. Note resultant collaterals (arrows) between bilateral intercostal, circumflex iliac, and epigastric arteries.
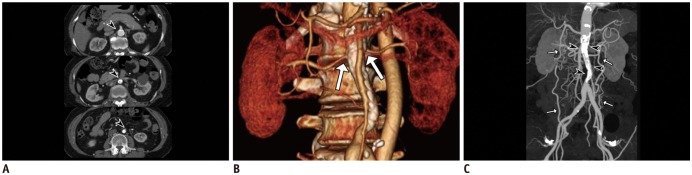
Fig. 8
Primary aldosteronism in 68-year-old male with uncontrolled hypertension, low level of plasma renin activity (0.2 ng/mL/hr) and elevated aldosterone level (47 ng/dL).
Incidentally detected right adrenal mass (arrowheads) (A), with attenuation coefficients of 8, 43, and 21 Hounsfield units at pre-contrast (upper column), portal venous (middle column), and delayed phases (lower column), respectively, making absolute percentage washout of 65% and relative percentage washout of 55%, consistent with adenoma. Right adrenal venous sampling (arrows) (B) confirmed high aldosterone level (13680 ng/dL). Subsequent right adrenalectomy yielded lesion that was confirmed to be adenoma.
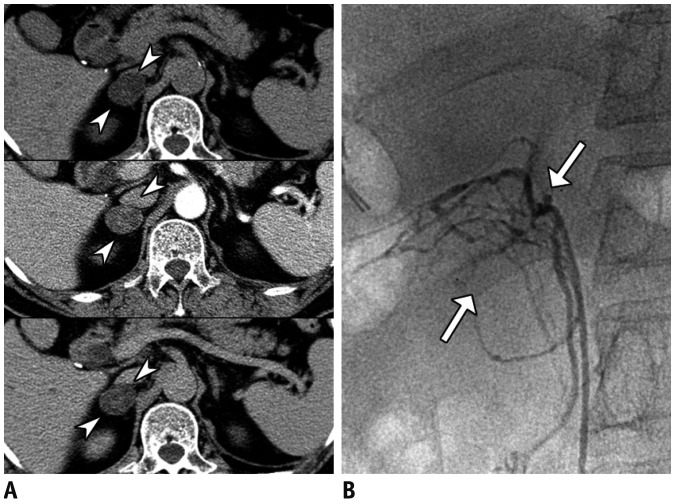
Fig. 9
Cushing's syndrome in 65-year-old female with hypertension, diabetes mellitus, and generalized weakness and markedly elevated adrenocorticotropic hormone (1260 pg/mL).
Axial CT scan (A) shows bilateral adrenal masses (arrowheads) with strong cortical enhancement. Whole-body PET (B) shows hypermetabolic adrenal masses (arrows), corresponding to CT. Lesions were surgically resected and were confirmed to be bilateral adrenal cortical hyperplasia.
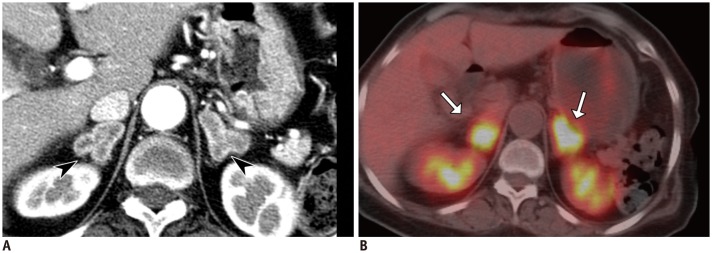
Fig. 10
Pheochromocytoma in 49-year-old male with hypertension, headache, and chest discomfort.
Coronal CT image (A) shows heterogeneously enhancing right adrenal mass with lobulated contour (arrowheads). Nuclear scintigraphy (B) shows high uptake of metaiodobenzylguanidine 28 hours after injection (arrow).
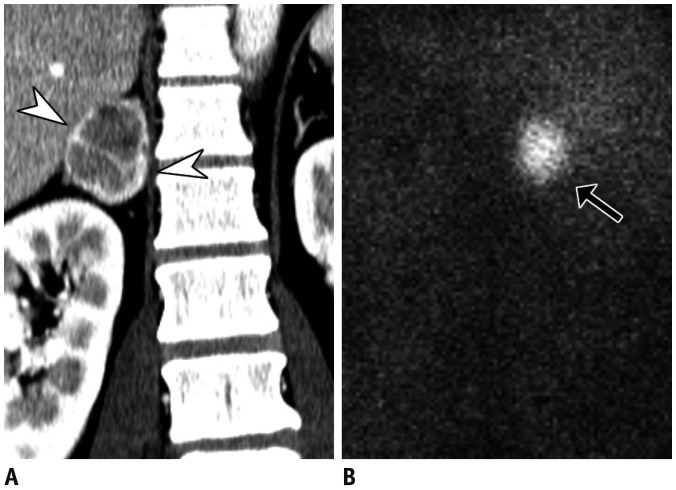
Fig. 11
Thyroid dysfunction in 79-year-old female with hypertension, depressed thyroid-stimulating hormone (< 0.01 µIU/mL), elevated T3 (4.4 ng/mL), and free T4 (> 5.54 ng/dL).
Color Doppler US (A) demonstrates diffusely enlarged left thyroid (arrowheads, right not shown) with heterogeneous parenchymal echogenicity, with so-called “thyroid inferno,” appearing as markedly increased vascularity. Coronal image of hybrid single positron emission CT (B) and maximum intensity projection (C) show increased uptake of 99mTc within both thyroid glands (arrows in B and C). Patient was diagnosed with Graves' disease and was started on methimazole.
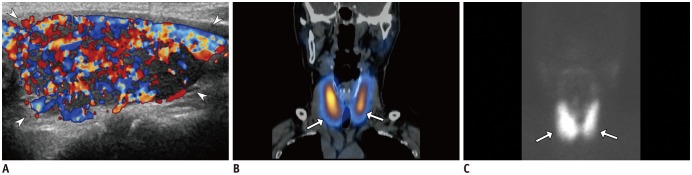
Fig. 12
Hyperparathyroidism in 47-year-old female with hypertension and generalized weakness.
Plain radiographs show multiple well-defined osteolytic lesions within femur (arrow) (A), presumably brown tumors, and subperiosteal bone resorption of phalanges of hand (B). Neck CT (C) shows inhomogeneous mass (arrowheads) located inferiorly to right thyroid. US (D) revealed hypoechoic mass in right lower parathyroid (arrowheads) containing focal calcification. Sestamibi scan (E) obtained two hours after radiotracer administration shows high uptake of Tc-99m in right lower neck (arrow). Lesion was later surgically confirmed to be parathyroid adenoma.
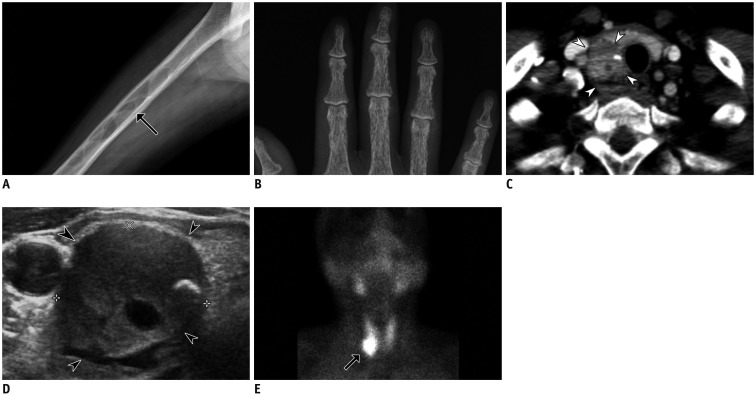
Table 1
Comparison of Various Diagnostic Imaging Modalities for Evaluation of Secondary Hypertension
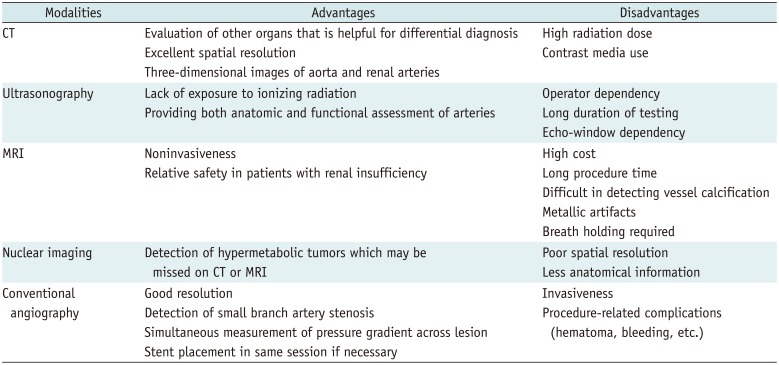
Table 2
Drug and Diet-Related Causes of Hypertension




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download