MATERIALS AND METHODS
We conducted this prospective single-center study at the endocrinology department of IRCSS AOU San Martino University-IST of Genoa (Italy) from September 2014 to September 2015. All patients signed an informed consent form that included a detailed explanation of the LA procedure and its purpose. Our Ethics Committee approved the LA treatment protocol.
Subjects
We evaluated patients with benign thyroid nodules, solid or nearly completely solid (with a liquid component not exceeding 30%), single or clearly detectable in a multinodular goiter, with a view to undergoing percutaneous LA (
3). These patients had either refused total or subtotal thyroidectomy, or had comorbidities that contraindicated surgery.
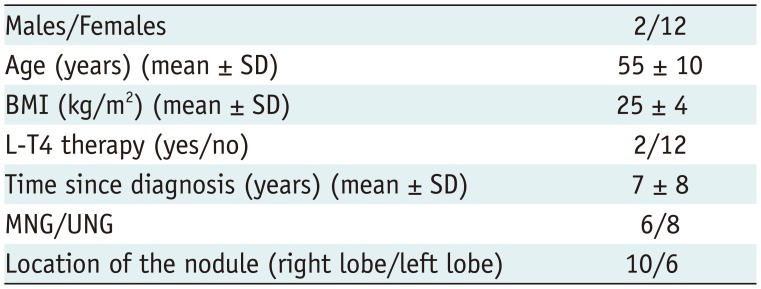
We enrolled 14 patients (12 females and 2 males) aged between 38 and 72 years (mean ± standard deviation [SD], 55 ± 10 years) with a single thyroid nodule (n = 8, 57%) or a predominant thyroid nodule in a goiter (n = 6, 43%). These nodules, which had been discovered 7 ± 8 years earlier, were located in the right lobe in 10 patients (71%) and in the left lobe in six patients (29%). No patient had undergone ethanol ablation of the nodule or radioiodine therapy. Two patients were on L-T4 replacement therapy for hypothyroidism at a mean dosage of 616 ± 124 µg/weekly.
Table 1 reports on patients' features.
Table 1
Patients Demographic Data
|
Males/Females |
2/12 |
|
Age (years) (mean ± SD) |
55 ± 10 |
|
BMI (kg/m2) (mean ± SD) |
25 ± 4 |
|
L-T4 therapy (yes/no) |
2/12 |
|
Time since diagnosis (years) (mean ± SD) |
7 ± 8 |
|
MNG/UNG |
6/8 |
|
Location of the nodule (right lobe/left lobe) |
10/6 |

Pre-Ablation Assessment and Protocol
Before performing LA, we excluded contraindications for the procedures, as recommended in the Consensus Statement of the Korean Society of Thyroid Radiology for RFA (
6). Before LA, patients underwent the following: a review of clinical data and physical examination; routine blood tests and thyroid function tests (free T3 [f-T3], free T4 [f-T4]; TSH); evaluation of anti-thyroid peroxidase antibodies (TPOAbs), calcitonin, thyroglobulin, electrocardiography; and a phoniatric examination. We evaluated the volume (mL) of the nodule undergoing LA by means of the ellipsoid formula: antero-posterior diameter × latero-lateral diameter × cranio-caudal diameter in millimeter (mm) × 0.52 / 1000. We also used this formula to calculate the total volume of each thyroid lobe (
7).
Before LA, patients underwent fine needle aspiration of the nodule on two separate occasions, to confirm benignity cytologically (Thy 2 in accordance with the British Thyroid Association (
8)). Only patients with a finding of benignity on both occasions underwent LA.
Patients filled out both a symptomatic visual analogic scale (VAS) that rated discomfort in the neck and a 13-scale Thyroid-specific Patient Reported Outcome (ThyPRO) QoL questionnaire validated for patients with thyroid disease (
9).
Clinical examinations, assessments of thyroid function and thyroid autoimmunity, and VAS scores were repeated after one week and 1, 3, 6, and 12 months. ThyPRO and ultrasound thyroid volume were evaluated after 1, 3, 6, and 12 months. We performed follow-up ultrasound examinations using a MyLab Five (Esaote®, Genoa, Italy) ultrasound scanner with a 7.5 MHz linear transducer (LA 523, Esaote®). We repeated phoniatric examination if symptoms related to chordal dysmotility appeared after the procedure.
Procedures and Methods
Laser ablation was performed in an out-patient regimen. Antiplatelet and anticoagulant therapies were suspended a week before the procedure. On the day of the procedure, patients were in a state of 8-hour fasting. After placement of a venous catheter, patients underwent intravenous infusion for over 30 minutes of ketorolac 20 mg (Tora-dol®, Recordati Spa, 10 mg/mL, Milan, Italy) and ranitidine 50 mg (Ranitidina®, 50 mg/5mL, S.A.L.F. Spa, Cenate Sotto, Italy) diluted in 100 mL of 0.9% saline solution. Subsequently, intravenous ketorolac 40 mg (Tora-dol® Recordati Spa, 10 mg/mL) and ranitidine 50 mg (Ranitidina® 50 mg/5 mL, S.A.L.F Spa) were administered in 500 mL of 0.9% saline for about five hours (during and after LA). In subjects allergic to ketorolac or in patients with moderate-to-severe renal impairment, premedication with intravenous paracetamol (500 mg) (Perfalgan®, 10 mg/mL, 50 mL, Bristol-Myers Squibb Srl, Roma, Italy) was carried out before and during the procedure. We placed patients on an operating table in the supine position with the neck hyperextended. Before LA, we performed a careful US examination to evaluate neck anatomy and ensure preservation of the “danger triangle” during procedures (
10). Local anesthesia with 2% lidocaine (Lidocaina Cloridrato®, 20 mg/mL, Monico Spa, Mestre, Italy) was carried out at the puncture site. If the patient did not tolerate pain during ablation, we reduced the power or turned it off for several seconds.
In three patients, we performed percutaneous drainage of cystic intranodular fluid before the procedure. LA was performed in a single session by a single operator. The operator had one year of experience with LA, four years of experience of thyroid RFA, and 22 years of experience with fine-needle aspiration biopsy, by means of 300 µm diameter fibers and a 21-Gauge Chiba-needle (ELESTA®, Calenzano, Italy). Procedures were performed by means of a commercially available US scanner (Echo-laser X4®, Esaote®) equipped with a 7.5 MHz linear transducer (LA 332, Esaote®) with a 1064 µm diode laser unit with a maximum of four laser sources, each with an individual energy emission setting and independent activation.
The operator was positioned at the patient's side and inserted from one to two fibers into the longitudinal length of the nodule, forming a shape matching the ellipsoid of thyroid nodule. The initial energy was delivered at 1200–1800 Joules per fiber with an output power of 2–4 W, starting at one centimeter from the bottom of the lesion. One or two 1-cm pull-backs were programmed to maximize the ablation volume in a single LA session. The number of fibers, their placement, the number of pull-backs and total energy delivered were tailored to the shape and volume of each thyroid nodule, as described by Baek et al. (
11). LA lasted 16 ± 2 minutes on average (range: 13–19 minutes), with a mean delivery of 4459 ± 1525 J (range: 1713–6203 J), one or two fibers (mean 1.7) and one or two pull-backs (mean 1.3). The energy load in J/mL was 340 ± 196 (range: 87–742 J/mL). Light irradiation was continuous, but was suspended in the event of pain or in order to reposition the fibers.
After LA, patients received a compressive bandage and ice to apply to the neck. Patients were allowed a light meal before being discharged in the early afternoon. All subjects received a domiciliary prescription for steroid administration (prednisone 25 mg for three days, 12.5 mg for three days, 6.25 mg for three days) and gastric protection, if not already ongoing.
Laboratory Tests
We measured TSH, f-T3, and f-T4 by ultra-sensitive chemiluminescence immunoassay (Cobas® e602, Roche Diagnostics, Milan, Italy). The normal ranges are: 0.27–4.2 mIU/L for TSH, and 2.76–7.07 pmol/L and 11.97–21.88 pmol/L for f-T3 and f-T4. We evaluated TPOAbs by means of DiaSorin (Saluggia, Italy); we regarded concentrations < 100 mU/L as negative. We measured calcitonin by chemiluminescence immunoassay (DiaSorin); in our laboratory, the normal value is < 10 ng/L. We performed automated complete blood counts using an ADVIA 2120 automated counter (Siemens Healthcare, Milan, Italy); coagulation tests on an automated BCS analyzer (Siemens Healthcare); and biochemical tests for the routine assessment of hepatic and renal function on the fully-automated Cobas® c701 platform (Roche Diagnostics).
Questionnaires
We evaluated the subjective benefits of LA several times during the study through a VAS with a score ranging from 0 (no symptoms/discomfort) to 10 (highest level of symptoms/discomfort). We assessed QoL before the procedure (baseline), and after 1, 3, 6, and 12 months, by means of ThyPRO, a validated questionnaire for thyroid diseases (
9), which consists of 13 scales with multiple-choice answers (0 = “none”; 1 = “slight”; 2 = “moderate”; 3 = “severe”; 4 = “very severe”). The ThyPRO scales concern the following aspects: goiter symptoms, hyperthyroid symptoms, hypothyroid symptoms, eye symptoms, tiredness, cognitive problems, anxiety, depression, emotional susceptibility, impaired social life, impaired daily life, impaired sex life, and cosmetic complaints.
We also evaluated a further scale, dubbed “general score.” It assesses answers to the question, “In the last 4 weeks, has thyroid disease had a negative effect on your QoL?” Lastly, we considered the mean values of the 13-scale score (
9).
Statistical Analysis
We present continuous data as means ± SD. We analysed all data by the GraphPad Prism for Windows (version 6.0, GraphPad Software, San Diego, CA, USA), by means of a non-parametric test. We assessed correlations between variables by means of the Spearman correlation. A p value < 0.05 indicated significance.
Go to :

DISCUSSION
The present paper reports our preliminary data on the use of a single session of LA to treat 14 benign thyroid nodules in 14 patients recruited over 12 months and followed up for 12 months.
We used standard procedures recommended by the Korean RFA Guidelines (
711), and we performed LA after excluding contraindications.
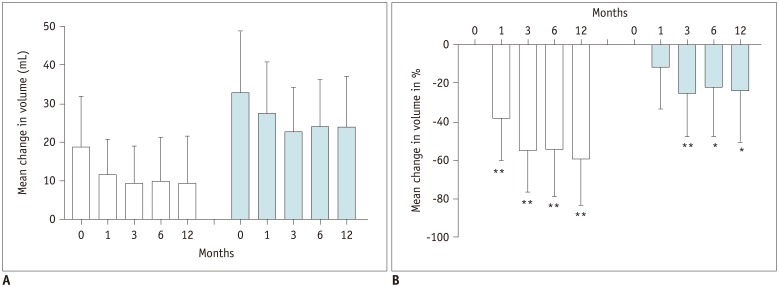
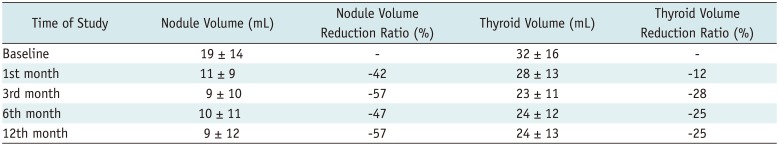
The mean reduction in the volume of our nodules appears to be similar or slightly lower than those observed in other centers: 58 ± 25%. At 12 months, the mean nodule volume reduction was −72 ± 11% in a study by Pacella et al. (
12), −84 ± 13% in a study by Achille et al. (
13), −59 ± 22% in a study by Papini et al. (
14), − 51 ± 11% in a study by Døssing et al. (
15), and −70 ± 16% in a study by Mauri et al. (
16).
The mean reduction in the volume of our nodules appears to be similar or slightly lower than that observed after a single session of RFA: −74 ± 12% in a study by Mauri et al. (
16), −69 ± 14% in a study by Cesareo et al. (
17), and 77 ± 3% in a study by Faggiano et al. (
18), −79.7% in a study of Baek et al. (
19), −84 ± 15% in a study of Jeong et al. (
20). We achieved more than 50% volume reduction at the 12 months in 86% of nodules. This result is comparable to, or better than, the data presented in a three-year multicenter prospective study (67.3%) (
14).
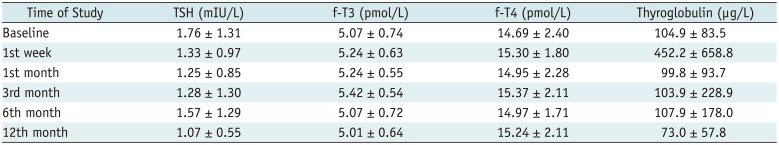
Laser ablation can lead to normalization of TSH in patients with hyperthyroidism with “hot” nodules (
21). The literature reports a change in thyroid function (hypo- or hyperthyroidism) in 2% of patients after LA (
12). By comparison, in our study that enrolled only euthyroid patients, LA did not lead to significant changes in thyroid function and preserved euthyroidism. This data confirms the safety of this procedure and may prompt percutaneous techniques to be preferred to surgery, which causes hypothyroidism in 100% of cases after total thyroidectomy and in 22% of cases after hemithyroidectomy (
2). In comparison studies between RFA and surgery, there were also no hypothyroidism patients after RFA (
2223).
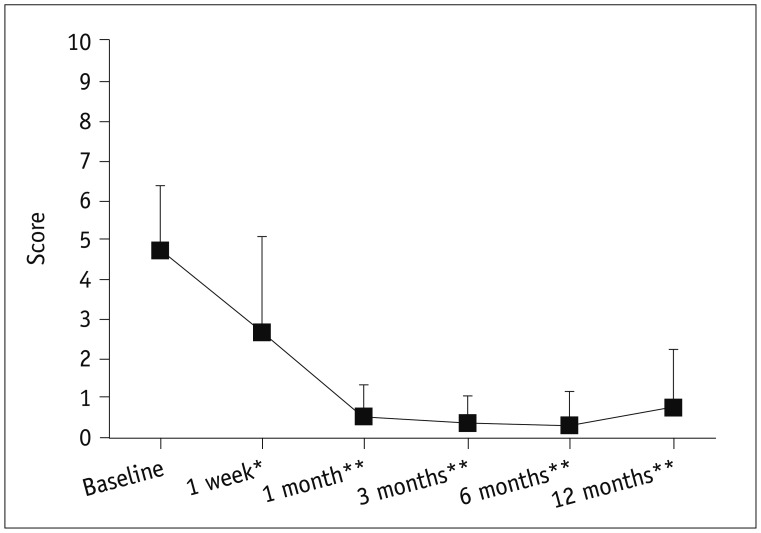
Most of our patients did not experience any problems either during or after LA. In our study, adverse events were few and mild; indeed, the main complaints were “discomfort in the neck” and “pain” during and after LA. If these symptoms started after LA, they resolved spontaneously in a few weeks; if they arose during LA, they were alleviated by reducing the energy delivered or turning off the fibers for several seconds. In any case, pain was mild, with the mean VAS score being 3.
Other adverse events in our experience were asymptomatic edema of the neck in one patient and a superficial hematoma in another patient; however, these resolved spontaneously. Our patients did not suffer other major adverse events, such as dysphagia, nodule rupture, pericapsular bleeding, vagal symptoms with bradycardia, or infections, which were reported in 0.5% of cases in a study by Pacella et al. (
12) on 1280 nodules treated with LA. In that study, the rate of major complications was lower in patients treated without local anesthesia or with no conscious sedation than in patients managed with local anesthesia and conscious sedation. Conversely, complaints of side effects were more frequent in subjects treated without local anesthesia (
12). In our protocol, we did not use conscious sedation, which probably explains why we observed no major complications and few adverse events. In our cohort of patients, we did not administer local sub-capsular anesthesia, but subcutaneous tissue anesthesia. Furthermore, we infused analgesic and anti-inflammatory drugs before and during the procedures and prescribed steroids for some days after discharge, in order to minimize complications. While these precautions probably helped to minimize complications, they did not completely alleviate pain and discomfort in the neck.
It should be borne in mind that proximity of the light to the capsule cause pain and discomfort in the neck. If patients complain of these symptoms during the procedure, the patients can thereby help the operator not to exceed the capsule of the treated nodule and minimize adverse events (
12). In any case, the complications of LA, and of other minimally invasive procedures, such as RFAs (
11), are fewer and milder than those of thyrodectomy, whether total or partial. The types and frequencies of adverse events of thyroid surgery are described in a recent review by a group of Indian surgeons (
24). A study by Wang et al. (
25) describes the prevalence of complications after conventional and endoscopic thyroidectomies as follows: transient laryngeal nerve palsy in 5.4%, transient hypocalcemia in 3.6%, bleeding in 0.5% and hematoma in 0.5% of cases. Furthermore, discomfort in the neck and chest was reported in 80% of patients on the third day, and pain in the neck and chest in 10% of patients one month after thyroidectomy (
25).
Many studies have reported improvements in neck discomfort and esthetic symptoms after LA. In a study by Valcavi et al. (
21), neck symptoms improved in 73% of patients, were unchanged in 23%, and worsened in only 4%; esthetic symptoms improved in 71% of patients, were unchanged in 24%, and worsened in 5%. Moreover, in a study by Pacella et al. (
12), neck symptoms improved in 10–49% of cases, and esthetic symptoms improved in 8–86% of cases. In addition, in a study by Achille et al. (
13), esthetic problems were completely resolved in 87% of treated patients, reduced in 9%, and unchanged in 2%, and pressure symptoms were resolved in 88% of cases. In our study, esthetic complaints were assessed by means of the cosmetic scale of ThyPRO, which revealed no improvement after LA. However, it should be noted that the baseline scores on this scale were very low in all our patients.
Quality of life is defined as a person's perception of his/her position in life, in the context of the culture and system of values in which he or she lives and in relation to his or her objectives, expectations, standards, and concerns (
26). In thyroid diseases, besides ThyPRO, QoL is often calculated by means of various questionnaires: the Short Form-36 (
27), the SF-12 Health Survey (
28), the Hamilton Depressive Scale (
29), and the Kellner Symptoms Questionnaire (
930).
While the Hamilton Depressive Scale (24-scale multiple-choice questionnaire) and the Kellner Symptoms Questionnaire (92-item questionnaire with Yes/No answers) focus more on the psychological aspects of QoL, the Short Form-36 (11-scale multiple-choice questionnaire), the slimmer SF-12 Heath Survey (12-item multiple-choice questionnaire), and ThyPRO analyze both physical and mental health (
927282930). We chose to utilize ThyPRO because it has been validated for thyroid diseases and could therefore provide a more detailed analysis of QoL in patients undergoing thyroid LA. Indeed, the 13 scales of ThyPRO investigate several aspects of life that may be impaired by goiter-related compression symptoms or discomfort in the neck, by esthetic alterations and by an altered thyroid function (both hypo- and hyper-thyroidism). As of this writing, no literature has reported on changes in QoL in patients treated with LA. However, ThyPRO has been used to assess QoL in numerous benign and malignant thyroid diseases. Regarding the use of questionnaires to evaluate changes in QoL in patients treated with thermal ablation for thyroid nodules, a study by Valcavi et al. (
31) reported a significant improvement in QoL. The study assessed QoL using the Physical Component Summary and Mental Component Summary of Short Form-12 after two years of follow-up and in 40 patients treated with RFA for benign thyroid nodules. In the that study, both the Physical Component Summary and the Mental Component Summary significantly improved after one year, and remained stable from year one to year two.
Our group recently published a study on changes in QoL, as assessed by means of ThyPRO after RFA of benign thyroid nodules. In that study, we did not find a significant improvement in ThyPRO scales, except for the “general score,” which improved after three months (
32). The cause of less improvement of ThyPRO scales in this study (
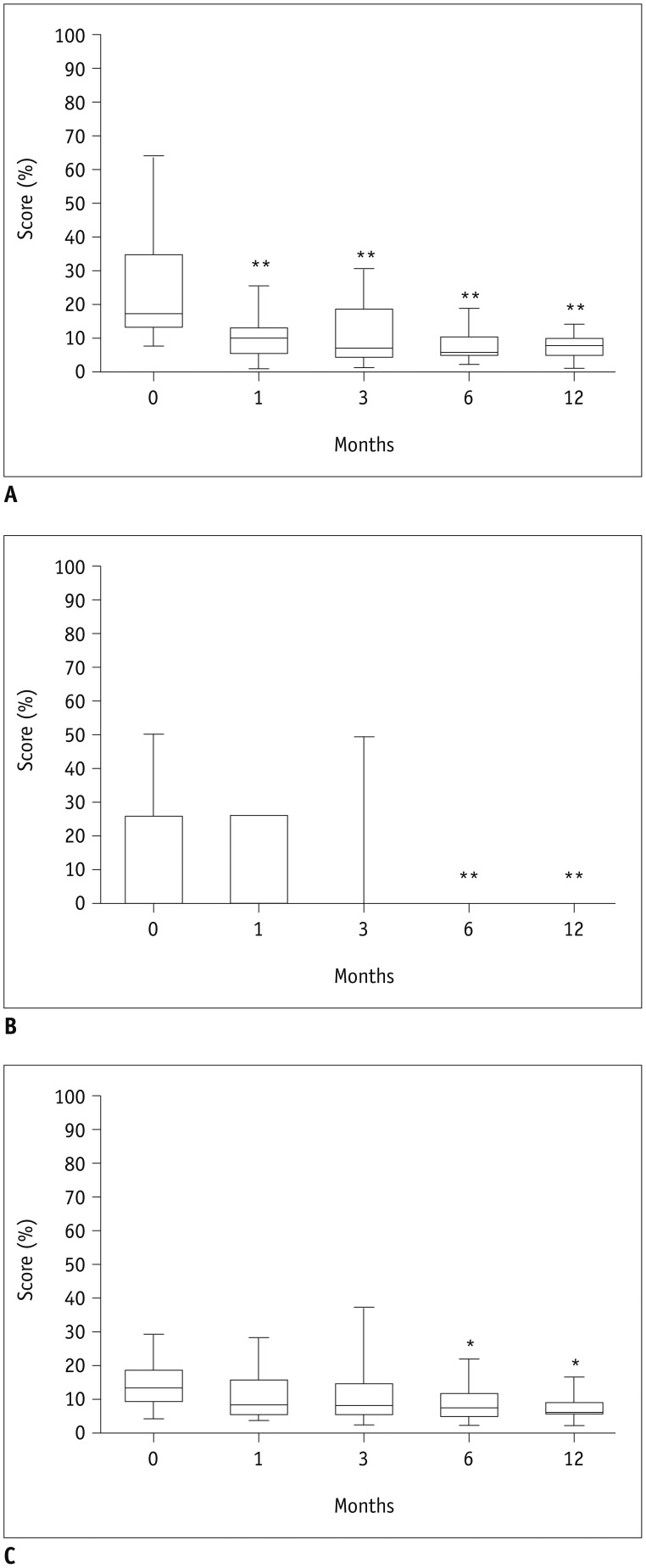
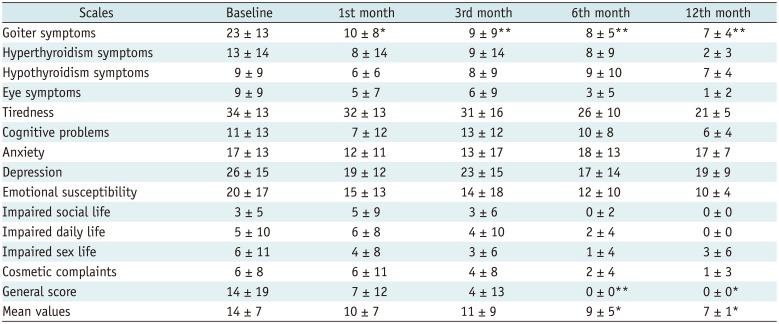
32) was probably induced by low volume reduction: the majority of patients showed less than a 50% volume reduction. Moreover, four patients showed increased nodule volume after RFA. The mean volume reductions of nodule was −18 ± 18%, −30 ± 24% and −27 ± 22% after RFA vs. −37 ± 23%, −53 ± 25% and −58 ± 25% in LA at 1, 6, and 12 months, respectively, although the features of nodules and inclusion criteria were the same in the two studies. In the present study, scores on the “goiter symptoms” and “general score” scales of the ThyPRO questionnaire showed a significant improvement from the first month and from sixth month onwards, respectively. The mean values of the scores on all 13 scales displayed a significant reduction from the sixth month onwards. In the present study, scores on each of the other scales did not change significantly. This could be due to LA does not affecting thyroid function. Indeed, changes in hyperthyroid symptoms, hypothyroid symptoms, eye symptoms, tiredness, cognitive problems, anxiety, depression and emotional susceptibility scales could be more closely related to changes in thyroid hormonal status.
Our study yielded more short-term data on changes in QoL than that of Valcavi et al. (
31). However, our cohort of patients was smaller and follow-up was shorter.
The main limitation of our study is its small population (n = 14) and short follow-up. Further studies, especially in a larger cohort of patients and over several years of follow-up, are necessary in order to confirm or disprove our data.
In conclusion, LA is effective in reducing both nodule volume and neck symptoms. Moreover, the procedure is safe. In comparison to surgery, adverse events are few and mild. LA is well-tolerated by patients and the VAS score improves significantly in all patients. In our cohort of patients, the goiter scale, the general score and the mean value of ThyPRO scores improved significantly a few months after LA for benign non-functioning thyroid nodules.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download