Abstract
Objective
To preliminarily evaluate technical feasibility of a dual-echo ultrashort echo time (UTE) subtraction MR imaging by using concurrent dephasing and excitation (CODE) sequence for visualization of iron-oxide enhancement in focal inflammatory pulmonary lesions.
Materials and Methods
A UTE pulmonary MR imaging before and after the injection of clinically usable superparamagnetic iron-oxide nanoparticles, ferumoxytol, was conducted using CODE sequence with dual echo times of 0.14 ms for the first echo and 4.15 ms for the second echo on 3T scanner in two rabbits concurrently having granulomatous lung disease and lung cancer in separate lobes. A mean ratio of standardized signal intensity (SI) was calculated for comparison of granulomatous lesion and cancer at first echo, second echo, and subtracted images. Lesions were pathologically evaluated with Prussian blue and immunohistochemistry staining.
Results
Post-contrast subtracted CODE images visualized exclusive enhancement of iron oxide in granulomatous disease, but not in the cancer (mean ratio of SI, 2.15 ± 0.68 for granulomatous lesion versus 1.00 ± 0.07 for cancer; p value = 0.002). Prussian blue and corresponding anti-rabbit macrophage IgG-staining suggested an intracellular uptake of iron-oxide nanoparticles in macrophages of granulomatous lesions.
T2* MR sequences are mainly used for depiction of iron deposition in liver or blood products, but are vulnerable to magnetic field inhomogeneity and motion artifact (1). Lung MRI has been limitedly used because of innate characteristics of the lung: a paucity of protons; a large magnetic-susceptibility difference at the interface between airspace and bronchovascular structure; and cardiopulmonary motions (2). Accordingly, observation of iron-oxide agents is fundamentally limited in the thorax if using T2* MR sequences that revealed intra-lesional iron-oxide uptake by macrophages (34). Such innate difficulties of lung MRI may be mitigated with ultrashort echo time (UTE) MRI (5). UTE MRI may be used for visualization of positive enhancement of iron-oxide agents by a dual-echo acquisition and subtraction instead of using T2* MR sequence (1). Concurrent dephasing and excitation (CODE) imaging is a three-dimensional radial gradient-echo-based UTE sequence and deflects most challenges of conventional UTE sequences, acquiring an asymmetric gradient echo and still offering echo time (TE) ≥ 0.14 ms on a clinical scanner (6). The purpose of our study was to preliminarily evaluate technical feasibility of dual-echo CODE subtraction imaging for visualization of iron-oxide enhancement in a focal inflammatory pulmonary lesion.
This experiment was approved by the Institutional Animal Care and Use Committee at Seoul National University Hospital (IACUC No. 13-0006-C2A0).
Concurrent non-caseating granulomatous lesions and lung cancer were induced in separate lobes in two rabbits. To establish concurrent lung cancer and non-caseating granulomatous lesion harboring pulmonary macrophages in lung parenchyma, an uncuffed polyvinylchloride tube with internal diameter of 2.5 mm and an outer diameter of 3.5 mm was used for tracheal intubation. Subsequently, a hydrophilic guide wire (Radifocus; Terumo, Tokyo, Japan) and a 4 Fr. angled guiding catheter (Davis; Cook, Bloomington, IN, USA) were applied through endotracheal tube on a conventional fluoroscopy for selection of lower lobes. A 1 mL of complete Freund's adjuvant was injected in the right lower lobe, subsequently followed by saline washing of 0.5 mL. A 1 mL of tumor suspension of VX2 carcinoma was inoculated in the left lower lobe. Mammals were allowed food and water ad libitum.
A dual-echo CODE imaging was conducted in two rabbits three weeks after induction of concurrent granulomatous lesion and cancer in separate lobes. MR images were acquired in a 3T MRI system (Magnetom Trio; Siemens, Erlangen, Germany) using a knee coil in supine position after sedation with intramuscular injection of 50-mg ketamine hydrochloride and 20-mg xylazine hydrochloride per kilogram of body weight. Detailed MR parameters for dual-echo CODE imaging were as follows: repetition time (msec), 8.00; TEs (msec), 0.14 for the first echo and 4.15 for the second echo; flip angle, 5°; field of view, 250 × 250 × 250 mm3; number of radial views, 60000; matrix size, 392 × 392 × 392; isotropic resolution = 0.64 mm3; radio-frequency pulse, sinc; pulse length (msec), 0.05. Baseline dual-echo CODE images were prior to intravenous administration of ultra-small superparamagnetic iron oxide nanoparticle (USPIO), ferumoxytol (Feraheme®, AMAG Pharmaceuticals, Lexington, MA, USA; 12 mg iron per kilogram body weight), into the marginal ear vein. Post-contrast dual-echo CODE images were acquired 24 hours after injection of ferumoxytol. Before and after contrast injection, the first echo images were subtracted by the second echo images to generate the subtraction images for enhancing positive contrast of iron-oxide. The second TE was empirically chosen for better enhancement of ferumoxytol after image subtraction.
After the acquisition of ferumoxytol-enhanced MR imaging, the rabbits were sacrificed with a lethal dose (90 mg/kg) of intravenously administered sodium pentobarbital (Pentothal; Choong Wae Pharmacy, Seoul, Korea). Bilateral lungs were isolated by one author and fixed with 10 percent formalin. Lung tissues were embedded in paraffin, and prepared consecutive sections (approximately 5-µm thick) in the axial plane with a 0.5-mm interval were stained with Prussian blue for identification of the iron-oxide nanoparticles. Immunohistochemistry staining was conducted by applying mouse monoclonal anti-rabbit macrophage IgG1 (RAM 11; DAKO Corp, Carpinteria, CA, USA) at a dilution of 1:500 in the section adjacent to a slide stained with Prussian blue to evaluate intracellular uptake of iron-oxide nanoparticles by macrophage.
CODE image reconstruction was conducted offline in MATLAB (R2011a; Mathworks, Natick, MA, USA) using fast Fourier transform via gridding. Two chest radiologists (with eight and 20 years of clinical experience in chest MR imaging, each) analyzed the CODE images in consensus. Round regions of interests were differently applied five times on consecutive baseline and post-contrast axial images within the margin of pulmonary lesions, not including vessel, airway, and adjacent normal parenchyma. For calculating signal-intensity (SI) ratio of those lesions (7), a mean SI ratio was calculated using the following formula: SI ratio = (SI lung lesion [postUSPIO] / SI muscle [postUSPIO]) / (SI lung lesion [preUSPIO] / SI muscle [preUSPIO]).
Nonparametric repeated measures ANOVA was used for comparison of mean SI ratio at 1st-echo, 2nd-echo, and subtracted images between granulomatous lung lesion and lung cancer. Statistical analysis was conducted using the SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
In two rabbits, the subtracted CODE images revealed heterogeneous positive iron-oxide enhancement in granulomatous lesions (Fig. 1). Mean SI ratios of second echo and subtracted images significantly differed between granulomatous lesion and lung cancer as follows: at first echo image, 1.10 ± 0.48 for granulomatous lesion versus 1.21 ± 0.18 for cancer (p value, 0.934); at second echo image, 0.51 ± 0.23 for granulomatous lesion versus 1.11 ± 0.16 for cancer (p value, < 0.001); at subtracted image, 2.15 ± 0.68 for granulomatous lesion versus 1.00 ± 0.07 for cancer (p value, 0.002). Prussian blue staining confirmed presence of iron-oxide nanoparticles in the granulomatous lung lesion, but not in lung cancer on the histopathologic specimen. Corresponding anti-rabbit macrophage IgG-staining suggests intracellular uptake of iron-oxide nanoparticles in macrophages. There were abundant macrophages in the granulomatous lesion, whereas few macrophages were found in the lung cancer.
Detection of iron-oxide agents in the thorax by dual-echo acquisition and subtraction is a unique imaging biomarker only achievable with UTE MRI. Although a T1 shortening effect of iron oxide was explored in lung parenchyma using UTE MRI before (8), previous investigation was limited to early phase of infection 24 hours after systemic bacteremia prior to forming focal pulmonary lesion. Histopathologic analysis of lung parenchyma revealed accumulation of iron oxide in lung parenchyma without formation of substantial edema or focal lesion. As they did not assess presence of macrophages in the pathologic examination, it was indistinguishable whether presence of an iron oxide agent during an early infection originated from extra-cellular accumulation by a regional hyperemia or from intra-cellular uptake by pulmonary macrophage.
Our preliminary study succeeded with in-vivo visualization of enhancement of ferumoxytol in the focal inflammatory pulmonary lesion without respiratory gating as CODE pulse sequence adopted radial acquisition that is robust against respiratory motion. A complete Freund's adjuvant induces a granulomatous lesion and attracts abundant macrophages (9). We validated intracellular uptake of iron oxide nanoparticles in macrophages by matching adjacent consecutive microscopic slides stained with Prussian blue and anti-rabbit macrophage IgG1. The number of rabbits was limited in this study but a lung cancer was used as a negative control in same rabbits. This allowed us to depict an exclusive accumulation of iron oxide in granulomatous lung disease, not in the lung cancer. Exclusive accumulation of iron oxide nanoparticle resulted from presence of pulmonary macrophages in inflammation, but not in the cancer. The amount of ferumoxytol (12 mg/kg) used in our study was higher than the typical dose of ferumoxytol (2–4 mg/kg) for human MRI (10). It was still lower than a standard dose of ferumoxytol for anemia treatment (1020 mg; equal to 14.6 mg/kg for an adult man of 70 kg). Further study is warranted to elaborate on our observation in a human.
In conclusion, a dual-echo UTE subtraction MR imaging using CODE sequence preliminarily succeeded in depicting an exclusive positive enhancement of clinically-usable superparamagnetic iron-oxide nanoparticle, ferumoxytol, in rabbits with focal granulomatous inflammatory lung disease. This new pulmonary MR imaging biomarker potentially provides an opportunity to differentiate a benign inflammatory lesion from malignancy.
Figures and Tables
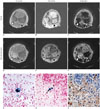 | Fig. 1Representative axial dual-echo CODE images on 3T MR scanner (A-F), and histopathologic Prussian blue, anti-rabbit macrophage IgG-stained images (G-I) of concurrent granulomatous lung lesion (right lower lobe, white arrowhead) and lung cancer (left lower lobe; black arrowhead) in rabbit.Images were obtained prior to intravenous administration of ferumoxytol (12 mg/kg) (A–C), 24 hours after injection (D–F). Baseline first (TE, 0.17 msec) and second echo (TE, 4.15 msec) images revealed similar mass-like consolidation in both basal lungs (A, B). When subtracted, there was identical signal nulling in granulomatous lung lesion and lung cancer (C). Ferumoxytol-enhanced MR images depicted higher SI at first echo and lower SI at second echo in granulomatous lung lesion if compared to those in lung cancer (D, E). When subtracted, there was obvious enhancement in granulomatous lung lesion whereas signal nulling in lung cancer (F). Prussian blue-stained slice revealed dense intracellular accumulation of iron oxide nanoparticles (× 400, G). Prussian blue and corresponding anti-rabbit macrophage IgG-staining slices revealed intracellular uptake of iron oxide nanoparticles in macrophages (black arrows) (× 400, H, I). CA = contrast administration, CODE = concurrent dephasing and excitation, SI = signal intensity, TE = echo time
|
References
1. Girard OM, Du J, Agemy L, Sugahara KN, Kotamraju VR, Ruoslahti E, et al. Optimization of iron oxide nanoparticle detection using ultrashort echo time pulse sequences: comparison of T1, T2*, and synergistic T1- T2* contrast mechanisms. Magn Reson Med. 2011; 65:1649–1660.
2. Bergin CJ, Pauly JM, Macovski A. Lung parenchyma: projection reconstruction MR imaging. Radiology. 1991; 179:777–781.

3. Raynal I, Prigent P, Peyramaure S, Najid A, Rebuzzi C, Corot C. Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: mechanisms and comparison of ferumoxides and ferumoxtran-10. Invest Radiol. 2004; 39:56–63.
4. Weissleder R, Nahrendorf M, Pittet MJ. Imaging macrophages with nanoparticles. Nat Mater. 2014; 13:125–138.

5. Johnson KM, Fain SB, Schiebler ML, Nagle S. Optimized 3D ultrashort echo time pulmonary MRI. Magn Reson Med. 2013; 70:1241–1250.

6. Park JY, Moeller S, Goerke U, Auerbach E, Chamberlain R, Ellermann J, et al. Short echo-time 3D radial gradient-echo MRI using concurrent dephasing and excitation. Magn Reson Med. 2012; 67:428–436.

7. Yoo RE, Choi SH, Cho HR, Jeon BS, Kwon E, Kim EG, et al. Magnetic resonance imaging diagnosis of metastatic lymph nodes in a rabbit model: efficacy of PJY10, a new ultrasmall superparamagnetic iron oxide agent, with monodisperse iron oxide core and multiple-interaction ligands. PLoS One. 2014; 9:e107583.

8. Strobel K, Hoerr V, Schmid F, Wachsmuth L, Löffler B, Faber C. Early detection of lung inflammation: exploiting T1-effects of iron oxide particles using UTE MRI. Magn Reson Med. 2012; 68:1924–1931.
9. Moore RD, Schoenberg MD. The response of the histiocytes and macrophages in the lungs of rabbits injected with Freund's adjuvant. Br J Exp Pathol. 1964; 45:488–497.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download