1. Lynch DA, Al-Qaisi MA. Quantitative computed tomography in chronic obstructive pulmonary disease. J Thorac Imaging. 2013; 28:284–290. PMID:
23748651.

2. Ostridge K, Wilkinson TM. Present and future utility of computed tomography scanning in the assessment and management of COPD. Eur Respir J. 2016; 48:216–228. PMID:
27230448.

3. Gould GA, Redpath AT, Ryan M, Warren PM, Best JJ, Flenley DC, et al. Lung CT density correlates with measurements of airflow limitation and the diffusing capacity. Eur Respir J. 1991; 4:141–146. PMID:
2044729.
4. Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000; 162(3 Pt 1):1102–1108. PMID:
10988137.
5. Sandek K, Bratel T, Lagerstrand L, Rosell H. Relationship between lung function, ventilation-perfusion inequality and extent of emphysema as assessed by high-resolution computed tomography. Respir Med. 2002; 96:934–943. PMID:
12418592.

6. Aziz ZA, Wells AU, Desai SR, Ellis SM, Walker AE, MacDonald S, et al. Functional impairment in emphysema: contribution of airway abnormalities and distribution of parenchymal disease. AJR Am J Roentgenol. 2005; 185:1509–1515. PMID:
16304005.

7. Grydeland TB, Thorsen E, Dirksen A, Jensen R, Coxson HO, Pillai SG, et al. Quantitative CT measures of emphysema and airway wall thickness are related to D(L)CO. Respir Med. 2011; 105:343–351. PMID:
21074394.

8. Mohamed Hoesein FA, de Hoop B, Zanen P, Gietema H, Kruitwagen CL, van Ginneken B, et al. CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax. 2011; 66:782–787. PMID:
21474499.

9. Mets OM, Murphy K, Zanen P, Gietema HA, Lammers JW, van Ginneken B, et al. The relationship between lung function impairment and quantitative computed tomography in chronic obstructive pulmonary disease. Eur Radiol. 2012; 22:120–128. PMID:
21837396.

10. Washko GR, Criner GJ, Mohsenifar Z, Sciurba FC, Sharafkhaneh A, Make BJ, et al. Computed tomographic-based quantification of emphysema and correlation to pulmonary function and mechanics. COPD. 2008; 5:177–186. PMID:
18568842.

11. Müller NL, Staples CA, Miller RR, Abboud RT. “Density mask.” An objective method to quantitate emphysema using computed tomography. Chest. 1988; 94:782–778. PMID:
3168574.
12. Gevenois PA, De Vuyst P, de Maertelaer V, Zanen J, Jacobovitz D, Cosio MG, et al. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996; 154:187–192. PMID:
8680679.

13. Madani A, Zanen J, de Maertelaer V, Gevenois PA. Pulmonary emphysema: objective quantification at multi-detector row CT--comparison with macroscopic and microscopic morphometry. Radiology. 2006; 238:1036–1043. PMID:
16424242.

14. Haruna A, Muro S, Nakano Y, Ohara T, Hoshino Y, Ogawa E, et al. CT scan findings of emphysema predict mortality in COPD. Chest. 2010; 138:635–640. PMID:
20382712.

15. Diaz AA, Bartholmai B, San José Estépar R, Ross J, Matsuoka S, Yamashiro T, et al. Relationship of emphysema and airway disease assessed by CT to exercise capacity in COPD. Respir Med. 2010; 104:1145–1151. PMID:
20385477.

16. Han MK, Kazerooni EA, Lynch DA, Liu LX, Murray S, Curtis JL, et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011; 261:274–282. PMID:
21788524.

17. Grydeland TB, Dirksen A, Coxson HO, Pillai SG, Sharma S, Eide GE, et al. Quantitative computed tomography: emphysema and airway wall thickness by sex, age and smoking. Eur Respir J. 2009; 34:858–865. PMID:
19324952.

18. Ashraf H, Lo P, Shaker SB, de Bruijne M, Dirksen A, Tønnesen P, et al. Short-term effect of changes in smoking behaviour on emphysema quantification by CT. Thorax. 2011; 66:55–60. PMID:
20978026.

19. Shaker SB, Stavngaard T, Laursen LC, Stoel BC, Dirksen A. Rapid fall in lung density following smoking cessation in COPD. COPD. 2011; 8:2–7. PMID:
21299472.

20. Mishima M, Hirai T, Itoh H, Nakano Y, Sakai H, Muro S, et al. Complexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A. 1999; 96:8829–8834. PMID:
10430855.

21. Gietema HA, Müller NL, Fauerbach PV, Sharma S, Edwards LD, Camp PG, et al. Quantifying the extent of emphysema: factors associated with radiologists' estimations and quantitative indices of emphysema severity using the ECLIPSE cohort. Acad Radiol. 2011; 18:661–671. PMID:
21393027.
22. Mitsunobu F, Ashida K, Hosaki Y, Tsugeno H, Okamoto M, Nishida K, et al. Complexity of terminal airspace geometry assessed by computed tomography in asthma. Am J Respir Crit Care Med. 2003; 167:411–417. PMID:
12554627.

23. Coxson HO, Whittall KP, Nakano Y, Rogers RM, Sciurba FC, Keenan RJ, et al. Selection of patients for lung volume reduction surgery using a power law analysis of the computed tomographic scan. Thorax. 2003; 58:510–514. PMID:
12775863.

24. Madani A, Van Muylem A, de Maertelaer V, Zanen J, Gevenois PA. Pulmonary emphysema: size distribution of emphysematous spaces on multidetector CT images--comparison with macroscopic and microscopic morphometry. Radiology. 2008; 248:1036–1041. PMID:
18710992.

25. Tanabe N, Muro S, Hirai T, Oguma T, Terada K, Marumo S, et al. Impact of exacerbations on emphysema progression in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011; 183:1653–1659. PMID:
21471102.

26. Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010; 7:32–43. PMID:
20214461.

27. Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005; 26:511–522. PMID:
16135736.

28. Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005; 26:720–735. PMID:
16204605.

29. Zach JA, Newell JD Jr, Schroeder J, Murphy JR, Curran-Everett D, Hoffman EA, et al. Quantitative computed tomography of the lungs and airways in healthy nonsmoking adults. Invest Radiol. 2012; 47:596–602. PMID:
22836310.


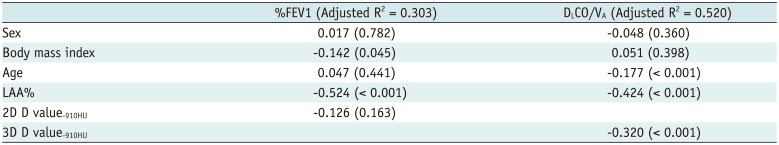
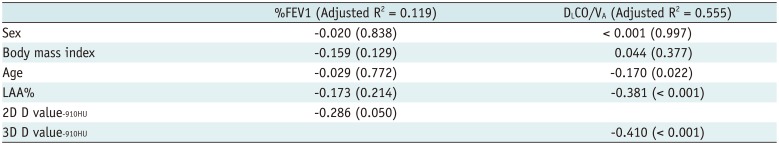




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download